Physicochemical Characteristics of Drinking Water Sources and Household Tape Water in Yabelo Town
| Received 10 Apr, 2023 |
Accepted 30 Aug, 2023 |
Published 09 Sep, 2023 |
Background and Objective: Drinking water distributed to a community must be free from pathogens and elevated levels of harmful substances at all times. So, monitoring the quality of drinking water before distributing it to the community is important. The study aimed to quantify the levels of selected drinking water parameters at the sources and Household tapes in Yabelo Town, Borena Zone, Oromia Regional State, Ethiopia. Materials and Methods: For this study, a total of twelve water samples were collected from two water sources and two household tapes. The concentration of heavy metals in the water samples was determined using a flame atomic absorption spectrophotometer and single beam UV-visible spectrophotometer. The AD8000 pH/mV/EC/TDS Meter was used to measure temperature, pH, EC (electrical conductivity) and TDS (total dissolved solids). Results: The study showed the levels of Total Dissolved Solids (TDS), Electrical Conductivity (EC), pH chloride and copper, except the levels of total hardness and manganese, were found to be lower than the Ethiopian Standard for Drinking Water Specification for all water samples. However, drinking water source 2 showed elevated levels of total alkalinity, calcium, magnesium and iron that exceed the maximum permissible limit set by the Ethiopian Standards Agency (ESA) and World Health Organization (WHO). Conclusion: In general, the result suggested that the drinking water provided to the Yabelo Town Community is potable and safe for consumption and doesn’t pose a health risk despite elevated levels of some physicochemical quality parameters that caused aesthetic problems.
| Copyright © 2023 Zigde and Begna. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Water is vital for human health and well-being. Water is needed in all life activities, especially for domestic and agricultural activities. Safe water represents affordable water, available in the required quantity as a beverage, for food preparation and for private hygiene and washing1. Safe water also signifies water that doesn't pose any significant health risk and it constitutes a major fulfilment source to fulfil the needs of basic hygiene that are necessary for curbing water-related diseases. However, the availability of safe water is restricted2. About, 3.0% of the whole globe’s water is considered fresh water and 2.97% of it is locked up in icecaps and glaciers. Only 0.03% of water is obtainable as surface and groundwater for human use3. In addition, the supply and distribution of the planet’s freshwater worldwide are uneven.
The availability of an adequate safe water supply is essential to human existence and it is recognized as a fundamental right of human beings that is placed next to oxygen as crucial for the existence of life. However, access to a clean, safe and adequate drinking water supply is still scarce in many developing countries and poses health risks associated with water-related diseases4. As a result, more than 700 million people, mostly living in developing countries, have no access to improved water sources and sanitation facilities5.
Although Ethiopia is the water tower of East Africa, water is still inaccessible in most parts of the country. Moreover, water quality is poor and often contaminated by human and animal faeces6-9. As a result of limited improved water availability, most rural population relies on unimproved water sources. It is estimated that about 57% of households have access to an improved drinking water source, with a higher proportion among urban residents (93%) than among rural residents (49%) showing a big disparity between urban and rural households in terms of access and types of services. Moreover, as access to improved sanitation facilities is very limited in rural areas, the majority of households defecate in the bush or open fields10. A study showed that only 42.2% of the household has access to improved drinking water sources and low status using improved sanitation facilities in the Oromia Region, Ethiopia11.
According to WHO/UNICEF5 and Simelane et al.10 improved drinking water sources are defined by the nature of their design and construction, have the potential to deliver safe water and include piped water, boreholes or tube wells, protected dug wells, protected springs, rainwater and packaged or delivered water. Drinking water sources such as ponds, lakes, rivers and open-dug wells are considered unimproved. In addition, drinking water sources that are poorly constructed or do not have any engineered facilities such as spring boxes, borehole capping and wells are also considered unimproved12. The use of unimproved water sources usually associated with the presence of indicator bacteria and elevated levels of harmful substances or pollutants10,13-18 could cause water contamination and lead to several diseases such as cholera, dysentery, salmonellosis and typhoid19,20. Waterborne diseases associated with these are attributed to the death of millions in developing countries every year21,22. As a result, deaths recorded due to poor quality of drinking water is very high in developing countries (99.8%)23 and about 1.7 million losses and 54.2 million disability-adjusted life-years worldwide are attributable to unsafe water and poor sanitation and hygiene problem24. Additionally, the study indicated that about one-fourth of the world’s population used unsafe water in 2010 and more than one-six world’s population used water from water sources with substantial hygienic hazards4.
In Ethiopia, from surface to improved sources, 35 million people have gained access to water from piped systems, protected hand pumps and springs. So, Ethiopia achieved the drinking water Millennium Development Goals (MDG) target of 57%, successfully halving the number of households without access to improved drinking water since 1990. In doing so over 52 million people in Ethiopia now have access to an improved drinking water source (within 1.5 km) as compared to only 6 million people in 199025. This achievement is primarily the consequence of significant improvements in access to drinking water supplies in rural areas. However, Ethiopia has a significant challenge to increase the water quality coverage to achieve the sustainable development goals (SDGs) aimed at universal access to safe water supply and sanitation26. Safe water supply includes the quality of water at the sources or drinking water must be free from pathogens and elevated levels of harmful substances at all times. So, the quality of drinking water can be compromised when it is contaminated by waste from various sources. The sources of water contamination could be geological, industrial and agricultural activities. These contaminants are further categorized as microorganisms, inorganics, organics and radionuclides. They can affect the quality of water and human health upon consumption before proper treatment27. Therefore, water quality control is a major strategy program in numerous parts of the world28.
Several scientific processes and methods have been developed to assess physicochemical parameters and biological characteristics in drinking water29. This physicochemical parameter includes pH, turbidity, Electrical conductivity (EC), total suspended solids (TSS), total dissolved solids (TDS) and heavy metals. These parameters can affect the drinking water quality if their values are higher than the standard values set by the WHO and other regulatory bodies28.
This study focuses on the assessment of selected physicochemical parameters in drinking water sources and taps water selected from households in Yabelo Town, one of the districts in Borana Zone, Oromia Regional State of Ethiopia. In addition, the levels of the selected physicochemical parameters will be compared with international guidelines and national standards for drinking water quality.
MATERIALS AND METHODS
Study duration: Sample preparation and analysis were carried out at Hawassa University Research Laboratory between June, 2022 and December, 2022.
Description of the study area: Yabello Town is the administrative seat of the Borana Zone and lies 570 km South of Addis Ababa. The zone covers 48,360 km2 of which 75% consists of lowland. The area is characterized by a semi-arid and arid kind of climate and people are predominantly involved in small-scale subsistence agriculture production and mainly livestock husbandry. Borana zone receives an average annual rainfall ranging from 350 mm to about 900 mm30,31.
The major and important source of water for domestic use in Yabelo town is groundwater and the town’s drinking water supply comes from seven borehole water sources. Currently, one of the borehole water sources is non-functional. The study focused on the assessment of selected physicochemical water quality parameters of drinking water samples taken from two water sources and household tap water. This is because the quality of the water generated from the water sources and distributed to the community is not checked. Furthermore, water scarcity and availability are always an issue in the town because the site suffers from frequent electrical cuts and low voltage that lead the community to shift to unimproved alternative water sources. This situation puts society at high risk of water-related and water-borne health problems.
Sampling and sample collection: Currently, the major and important source of water for domestic use in Yabelo Town comes from seven borehole water sources in which one of the borehole water sources is non-functional. The water from the two boreholes is directly stored in a storage reservoir and distributed to the community through a network of pipes. The water from the rest of the four boreholes’ water sources is directly distributed to the community using different piped networks. Therefore, water samples were taken from the source and household taps or at the point of collection to test the quality of the water being consumed by the community. In this study, water samples were collected from the out late of the reservoir, from one of the four borehole water sources and two tap water samples from nearby households. Therefore, a total of four water samples were collected purposefully for analysis. All water samples were collected in triplicate in clean 1000 mL plastic bottles and transported to the Hawassa University Research Laboratory. Then, all samples were acidified with concentrated HNO3 by adding 2.0 mL of concentrated HNO3 (69-72%) and then kept in the refrigerator at a low temperature of about 4°C before analyses.
Digestion of water samples: Nitric acid digestion techniques were used as it is adequate for clean samples such as drinking water to reduce interference by organic matter and to convert metals associated with particulates to a form (usually the free metal) that can be determined by atomic absorption spectrometry. In this method, 100 mL of well-mixed, acid-preserved drinking water samples were transferred to a flask. In a hood, 2 mL concentrated HNO3 (69-72%) was added. Then, the flask was covered with a ribbed watch glass to minimize contamination. The content of the flask was subjected to a slow boil and evaporated on a hot plate to the lowest volume possible (about 10 to 20 mL) before the sample dries. The flask was removed from the hotplate (Spectrum Hot Plate/Magnetic Stirrer, Spectrum chemical manufacturing & distribution, 14422 S. San Pedro Street Gardena, CA 90248-2027 USA) and the wall and the watch glass cover were washed down with distilled water into the flask and then filtered to a 100 mL volumetric flask. Then, the contents of the flask were cooled, diluted to mark and mixed thoroughly. The portion of the solution was used for required total metal determinations. All samples were digested during the same procedure. Furthermore, a reagent blank was prepared with the same acids and subjected to the same digestion procedure as the sample to correct for impurities present in acids and reagent water.
Apparatus and instrument: Different Instruments and apparatuses have been used to analyse the concentration of heavy metals in the water samples. Round bottom flasks (250 mL) for the digestion of water samples, borosilicate volumetric flasks (50 and 100 mL sizes) were used for the dilution of samples and preparation of metal standard solutions. Measuring cylinders (DURAN, Germany), pipettes (PYREX, USA), microliter pipettes (20-50 and 100-1000 μL size, DRAGON MED, Shangai, China) were employed for measuring different quantities of volumes of sample solution, acid reagents and metal standard solutions. Whatman filter paper No. 42 was used to filter digested water samples. Concentrations of metals were determined by a flame atomic absorption spectrophotometer (BUCK SCIENTIFIC, Model 210VGP AAS, USA) equipped with a deuterium background corrector and hollow cathode lamps with a flame produced from air-acetylene as oxidant-fuel or flame atomizer. A single-beam UV-visible spectrophotometer (CECIL, Et 1021 1000 series, serial no 121-789) was used for the determination of iron in water samples. AD800pH/mV/EC/TDS meter (Adwa Instruments, Hungary) was used to measure temperature, pH, EC and TDS.
Chemicals and reagents: All reagents and chemicals used in this study were analytical grades. Nitric acid, 69-72% HNO3 (Riedel-de Haen) was used in the sample digestion steps. Stock standard solutions of concentration 1000 mg L–1 of the metals Ca, Mg, Cu, Mn, Co, Cr, Ni and Pb (BUCK SCIENTIFIC, PURO-GRAPHIC) as nitrate salt standard solutions were used to prepare intermediate standard solutions. Ammonium iron (II) sulphate [Mohr’s Salt, (NH4)2Fe(SO4)2•6H2O], Sodium Acetate (CH3CO2Na), 99% Hydroxylamine hydrochloride (NH2OH•HCl), 1,10-Phenanthroline (C12H8N2•H2O) and 98% concentrated Sulphuric acid (H2SO4) was used. The HCl (37%) and phenolphthalein were used for the determination of total alkalinity, AgNO3, NaCl and K2CrO4 were used for the determination of chloride content of the water sample, Ethylene Diamine Tetraacetic Acetic acid (EDTA), Eriochrome black T (EBT), MgSO4, NH3/NH4Cl buffer for the determination of the hardness of the water sample. Distilled water was used for all dilutions. Distilled water was used for the dilution of the sample and intermediate metal standard solutions and for rinsing glassware and sample bottles.
Determinations of temperature, pH, electrical conductivity and Total Dissolved Solids (TDS): Temperature, pH, electrical conductivity and TDS were measured using an AD8000 pH/mV/EC/TDS/Temp meter. Adwa intruments, Alsókikötõ sor 11, 6726 Szeged, Hungary.
Determination of total alkalinity: Alkalinity is a useful measure of the capacity of water to resist acidification from acid precipitation. The presence of carbonate, bicarbonate and hydroxide ions impart
the alkalinity of natural or treated waters. In this method, 100 mL of water sample will be pipetted into a 250 mL conical flask and three drops of methyl orange indicator will be added to the sample. Then, the sample solution will be titrated with 0.10 N HCl solutions from the burette until the indicator color changes from yellow to red. The volume of acid used corresponds to the sum of carbonate and bicarbonates present in an aqueous solution. The titration will be carried out in triplicate. If the volume of titrant used was recorded as V2 (mL), then the Total alkalinity is calculated32,33:
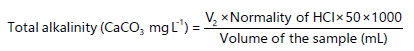 |
Determination of total hardness (TH): Hard waters are the waters that require a considerable amount of soap to produce foam and also produce scale in hot water pipes, heaters, boilers and kettles. The hardness index is used to measure the concentration of Ca2+ and Mg2+ ions in water. Chemically, the hardness index is defined as the sum of the concentration of Ca2+ and Mg2+. Hardness is traditionally expressed as the mass (in mg) per liter of water calcium carbonate that contains the same number of positive (2+) ions. In this study, hardness is measured by titration of a water sample against standard EDTA at pH 10 and using an indicator (EBT) solution to signal the endpoint34. Consequently, the hardness of water can be calculated by measuring the amount of EDTA consumed by a water sample and relating this to the concentration of dissolved calcium and magnesium ions. In this method, 100 mL of each of the water samples will be pipetted out in a washed 250 mL Erlenmeyer flask. Then, 10 mL of ammonia/ammonium chloride buffer solution and three drops of EBT indicator solution will be added into the flask, which turns the color of the solution wine red. This solution will be titrated against previously standardized 0.01 M EDTA solution taken in the burette until the color changes from wine red to faint violet to pure blue or sky blue which indicates the endpoint. The final reading of the burette is noted and the titration is repeated to get a concordant value. Then, the total hardness is calculated as34:
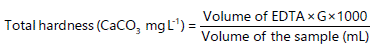 |
Where:
| G | = | mg CaCO3 is equivalent to 1.0 mL of EDTA titrant at the calcium indicator endpoint |
Determination of chloride: Chloride is one of the major inorganic ions in water and wastewater. In potable water, the saltiness is variable and dependent upon both the concentration of chloride ions and the presence of other ions (Na, K, Ca and Mg,). There are many potential sources of chloride in natural and drinking waters. The chloride concentration is higher in wastewater than in raw water because sodium chloride is a common article of diet and passes unchanged through the digestive system. Along the sea, coast chloride may be present in high concentrations because of the leakage of salt water into the sewage system. In addition, high chloride contents may harm metallic pipes and structures as well as growing plants. One of the analytical procedures to determine the chloride concentration is Mohr’s titration35. In this method, Silver nitrate (0.0141 M) is prepared by dissolving 2.4 g of powdered AgNO3 with distilled water and then it was made up to the mark in a 1000 mL volumetric flask. The silver nitrate solution was transferred into a well-rinsed 50 mL burette. Then, 50 mL of each water sample was measured using a measuring cylinder and 1.0 mL of potassium chromate indicator was measured with a dropper and added to the sample. In the conical flask, the mixture was titrated with the silver nitrate solution from the burette to a reddish-brown precipitate that signals the endpoint. Titration will be done thrice and the average
volume of AgNO3 will be recorded. A blank value was established by titration with 50 mL of distilled water. Calculation of the concentration of chloride ions is done using the following formula35:
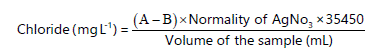 |
Where:
| A | = | Volume of titrant used for the water sample | |
| B | = | Volume of the titrant used for the blank (distilled water) |
Determination of Ca, Mg, Cu, Zn, Cd, Mn, Co, Cr, Ni and Pb: The determination of metals (Ca, Mg, Cu, Mn, Co, Cr, Ni and Pb) will be carried out using a Flame Atomic Absorption Spectrometer (Buck Scientific Model 210 AA, Buck Scientific, Inc.). Five working standard solutions of each metal will be prepared from certified stock standard solution (1000 mg L–1) in distilled water. Then, the working standard solutions for each metal will be aspirated and absorbance will be recorded. Then, the absorbance values will be plotted as a function of the concentration of working standards to generate a calibration curve. Once the calibration curve is linear and satisfied, the sample solution will be aspirated and their concentration is read directly from the Flame atomic absorption spectrometer (FAAS).
Determination of Fe: The determination of iron will be carried out using a Series 1000 Single Beam UV/Visible Spectrophotometer (CECIL Instruments Ltd.). In this method, iron is determined by its reaction with 1, 10-phenanthroline to form an orange-red complex. This complex has a stable colour at a pH range of 2-9. Below pH 2 the colour is weak and develops slowly. The complex obeys Beer-Lambert law in its absorption and remains unchanged for many months. The 1, 10-phenanthroline is a weak base and in acidic solutions, the essential species is the phenanthoium ion PhenH+ so the complex formation reaction can be represented by the equation:
Fe+2(aq)+3 phenH+(aq)→Fe(Phen)+2(aq)+3H+(aq) |
The ferric ions in the sample can be reduced by hydroxylamine hydrochloride or hydroquinone. The pH range is 2-9 but 3.536 is recommended to prevent the precipitation of various ion salts like phosphate. The complex formed absorbs at a wavelength of 510 nm theoretically. Therefore, iron will be measured by this method in all samples under study which will be in a liquid form.
Preparation of stock standard solution: As 1.2 M sodium acetate buffer, 5.0×10–3 M 1, 10-phenanthroline, 0.29 M Hydroxylamine Hydrochloride (NH2OH•HCl) and 5.1×10–4 M iron stock standard solutions were prepared.
Preparation of iron stock standard solution: Accurately 0.200 g of reagent grade [(NH4)2Fe(SO4)2•6H2O] (Mohr’s salt) was weighed and transferred quantitatively into a 1000 mL volumetric flask containing 50 mL distilled water. Then, 20.0 mL of 2.0 M sulphuric acid was added to the flask to dissolve the Mohr’s salt completely. The solution was thoroughly mixed and diluted to the mark with distilled water and the concentration of the solution was calculated to be 5.1×10–4 M iron stock standard solution.
Preparation of ironworking standard solutions: Five standards and one reference blank were prepared using a series of six 50 mL volumetric flasks. Into each flask, 0.5, 1, 1.5, 2, 2.5 and 0.0 mL of iron stock standard solution were added, respectively. Then, 1.0 mL hydroxylamine solution, 5 mL sodium acetate solution and 5.0 mL 1,10-phenanthroline solution were added to each of the six flasks. The contents of the flasks were mixed and filled to the mark with distilled water. The solutions were allowed to stand for
about 20 min before the absorbance of each solution was measured. Similarly, 5.0 mL of each digested sample was added into a separate 50.0 mL volumetric flask and all the three reagents 1.0 mL hydroxylamine solution, 5 mL sodium acetate solution and 5.0 mL 1,10-phenanthroline solution were added into each flask. Then, the contents of the flasks were mixed and filled to the mark with distilled water. After about 20 min, the sample solutions were ready for analysis.
Evaluation of analytical methods: Method validation is the process of providing that the analytical method is acceptable for its intended purpose appropriate quality assurance procedures and precautions were carried out to ensure the reliability of the results. Distilled water and analytical-grade reagents were used throughout the study. Reagent blank determinations were used to correct the instrument readings. For validation of the analytical procedure, a recovery study was carried out by spiking and homogenizing several already analysed samples with varying amounts of standard solutions of the metals.
Flame atomic absorption spectroscopy (FAAS) operating conditions: In this study, a total of six metals for each water sample were analysed using FAAS with an external calibration curve after the parameters such as burner and lamp alignment, slit width and wavelength adjustment were optimized for the maximum signal intensity of the instrument. For each metal (Ca, Mg, Cu, Mn, Co, Cr, Ni and Pb), the respective hollow cathode lamp was inserted into the atomic absorption spectrophotometer and the solution was successively aspirated into the flame. Three replicate determinations were carried out for each sample. The operating conditions of the instrument employed for each analyte are shown in Table 1.
FAAS calibration: Calibration curves were prepared to assess the concentration of metals in water sample solutions. For the instrument calibration, intermediate standard solutions containing 10 mg L–1 were prepared in a 100 mL volumetric flask from the stock standard solutions that contained 1000 mg L–1 of each metal. Then the intermediate standards were diluted with deionized water to obtain four working standards of each metal of interest for calibration purposes. A rinse blank (deionized water) was used to flush the uptake system to reduce memory interference. Hence, the instrument was calibrated using four working standards. The concentration of working standards and values of the correlation coefficient of the calibration graph for each metal are listed in Table 2.
UV-visible spectrophotometer calibration: Five working standard solutions of iron-phenanthroline complex and one reference blank solution were prepared in six 50 mL volumetric flasks (Table 2). Then, the absorbance of one of the standards was scanned at various wavelengths ranging from 400 to 520 nm and the absorption spectrum of the iron-phenanthroline complex was obtained by plotting absorbance versus wavelength. From the spectrum, the wavelength of maximum absorption (λmax) for the iron phenanthroline complex was determined. Then, the Uv-Vis spectrophotometer was adjusted to λmax = 510 nm and the blank was used to AUTHOZERO the UV-Vis spectrometer. Then, the absorbances of the five standard solutions were determined and a calibration curve was constructed by plotting absorbance versus concentration.
| Table 1: | Instrumental operating conditions for determination of selected metals in water sample using FAAS | |||
| Elements | Ca |
Mg |
Cu |
Mn |
Co |
Cr |
Ni |
Pb |
| Wavelength (nm) | 422.7 |
285.2 |
324.7 |
279.5 |
240.7 |
357.9 |
239.7 |
283.2 |
| Slit width (nm) | 0.7 |
0.7 |
0.7 |
0.7 |
0.2 |
0.7 |
0.2 |
0.7 |
| Lamp current (mA) | 2 |
1 |
1.5 |
3 |
4.5 |
3 |
3 |
2 |
| Table 2: | Concentration of working standards for each metal analysed using FAAS | |||
| Metals | Concentration of the standards (mg L–1) |
| Ca | 1.0, 2.0, 4.0, 6.0 |
| Mg | 1.0, 2.0, 4.0, 6.0 |
| Cu | 0.1, 0.3, 0.6, 1.0 |
| Mn | 0.25, 0.5, 1.0, 1.5, 2.0 |
| Co | 0.5, 1, 3, 4 |
| Cr | 1, 2, 3, 5 |
| Ni | 1, 2, 3, 6 |
| Pb | 0.1, 0.2, 0.3, 0.6, 1.0 |
| Fe | 5.1×10–6 M, 1.0×10–5 M, 1.5×10–5 M, 2.0×10–5 M, 2.5×10–5 M |
Method detection limit (MDL): Blank samples were prepared for each sample using a similar procedure for sample digestion. The method detection limits for each metal were estimated by digesting six analytical blanks for each water sample. Each blank solution was run with FAAS for the level of the metal in a similar manner as the samples and triplicate readings were recorded. Then, the standard deviations of the blanks’ concentration were calculated and used to calculate the method detection limit as follows37:
MDL = 3×Standard deviation of the blank |
Method validation (recovery experiment): The accuracy of the analytical procedure for sample analysis was tested by spiking experiment as there were no available certified reference materials and calculating the recovery (%)37. In this experiment, one sample was randomly selected, spiked and digested in triplicate following the digestion procedure used for water samples digestion. The digested spiked samples were analysed for their respective metal content using FAAS. Thus, the concentrations for the “spiked” and “unspiked” samples were used to calculate the recovery (%) as follows37:
Spiking of water samples: To recovery (%) of each metal was done using a 1000 mg L–1 stock standard solution of each metal. The selected water sample was spiked with 50 μL of Ca, Mg, Cu, Mn, Co, Cr, Ni and Pb standard solutions at once that contained 100 mL of the water sample. The spiked samples were digested in the same manner as the unspiked water samples. All the spiked samples were digested in triplicate following the same digestion procedure used for unspiked samples. Then, the digested spiked samples were analyzed for their respective metal content similarly using FAAS as the unspiked samples. Similarly, 1.0 mL of mg L–1 of Fe was spiked in 100 mL of each water sample and was digested in the same manner as the unspiked water samples. Then, the spiked samples were analysed similarly using a UV-visible spectrophotometer as the unspiked samples.
Precision of the method: The precision of the method expresses the variations within the same laboratory, when the same sample is analysed by the same procedure, by instrument and/or by different analysts in a different time interval and was evaluated by calculating the percentage of relative standard deviation (RSD) of the same samples digested and analysed by the same procedure and by the same instrument.
Statistical analysis: The data analysis for all parameters was done by using SPSS version 16.0, Origin 8 and the Microsoft Excel program. The Mean Standard Deviation, RSD (%) and One-way Analysis of Variance (ANOVA) were done using the above statistical analysis tools. A 95% significance level was used for all statistical calculations.
RESULTS AND DISCUSSION
Calibration curve for metals analysed using AAS and UV-Vis spectrophotometer: The correlation coefficients for the calibration curves for all the selected metals analysed using FAAS were greater than or equal to 0.999 which assured the linearity of instrumental response for individual metals or these correlation coefficients showed that there was a very good correlation (linear relationship) between concentration and absorbance. The equation of the graph and its R2 value for each metal are shown in Table 3.
The concentration of zinc and cadmium was not determined in all water samples due to the low output of lamp energy of their respective hollow cathode lamps. However, iron was determined using a UV-Vis spectrophotometer and the correlation coefficients for the calibration curve for iron were greater or equal to 0.9978 which assured a very good linear relationship between absorbance and concentration. The slope of the calibration graph of iron and the path length (1 cm) of the cuvette were used to calculate the value of the molar absorptivity of the complex. It was found to be 11,043 L mol–1 cm–1. This value of the molar absorptivity of the iron complex was lower than the value reported in the literature, 11,100 L mol–1 cm–1 36 which resulted in a -0.51% error which might be due to systematic errors introduced during sampling, preparation and analysis.
Method detection limits (MDL) and instrumental detection limits (IDL) values: The method detection limit values for the selected metals analysed were above the instrumental detection limit values listed in the manufacturer’s installation, operation and maintenance manual. This showed that the concentration of the selected metals in the samples analysed was quantified with an acceptable degree of confidence using the instrument-flame atomic absorption spectrometer.
Results of the recovery experiment
Recovery (%) for iron: The results of the recovery experiment for iron determination in the spiked water samples are shown in Table 4.
The results of percentage recoveries for iron determination ranged from 95.6-111.4%. This suggested that the percentage recovery of iron in the selected water samples was within the acceptable range (80-120%) and hence the sample digestion procedure and method of analysis used were accurate.
Recovery (%) for metals analysed using AAS: The results of the recovery (%) experiment for the selected metals are shown in Table 5.
Recovery (%) values for all metals lie within the range 104.7-112.7%, which is in the acceptable range (80-120%) suggesting that the digestion procedure used and the method of analysis were accurate and valid. Since the concentration of Co, Cr, Ni and Pb in the water samples analysed were below the detection limit, a recovery experiment was not carried out for these metals.
Results of temperature, pH, electrical conductivity and total dissolved solids: The values of the physicochemical parameters such as temperature, pH, electrical conductivity and total dissolved solids for the selected drinking water sources and household water samples were shown in Table 6.
Total dissolved solids (TDS): Total dissolved solids can be described as inorganic salts and small amounts of organic matter present in solution in water. A TDS level below 1000 mg L–1 is acceptable according to CES-5838. The TDS level of drinking water source 1 (378.2 mg L–1) is lower than drinking water source 2 (592.8 mg L–1). However, these values of TDS were below the recommended value of 1000 mg L–1.
Electrical conductivity (EC): The EC is the determination of the capability of a material to pass electrical current and it depends on the availability of inorganic dissolved solids such as chloride, nitrate, sulphate, phosphate anions or sodium, magnesium, calcium, iron and aluminium cations. The EC is a measure of Total Dissolved Solids (TDS) i.e., it depends upon the ionic strength of the solution. An increase in the concentration of dissolved solids increases the ionic strength of the solution. The recommended level by WHO is 500 μS cm–1 39, while the Australian standard considers electrical conductivity of <800 μS cm–1 to indicate good quality. The EC value of source 1 was 873.7 μS cm–1 and the EC value of source 2 was 1335.3 μS cm–1, which indicated the presence of higher levels of dissolved minerals in both water sources. The value of EC found for both sources was above the recommended value of 500 μS cm–1 and Australian drinking water standards. The relationship between TDS and EC was also observed in this study. The higher TDS concentration indicates an increase in the electrical conductivity of water as shown in Table 6.
| Table 3: | Calibration graph equation and R2 value | |||
| Metal | Calibration equation | R2 value |
| Ca | Y= 0.03x+0.006 | 0.9999 |
| Mg | Y = 0.0015x+0.0003 | 0.9979 |
| Cu | Y = 0.0449x+0.0005 | 0.9994 |
| Mn | Y = 0.025x+0.0002 | 0.9998 |
| Co | Y = 0.0645x-0.0061 | 0.9991 |
| Cr | Y = 0.0695x-0.0079 | 0.999 |
| Ni | Y = 0.0241x+0.0157 | 0.999 |
| Pb | Y = 0.045x+0.0004 | 0.9993 |
| Fe | Y = 11043x+0.0149 | 0.9978 |
| Ca: Calcium, Mg: Magnesium, Cu: Copper, Mn: Manganese, Co: Cobalt, Cr: Chromium, Ni: Nickel, Pb: Lead, Fe: Iron and the significance level, α is equal to 0.05 | ||
| Table 4: | Iron recovery result for the spiked water samples | |||
| Sample type spiked | Fe Conc. in the unspiked sample |
Amount of Fe spiked |
Fe Conc. in a spiked sample |
Recovery (%) |
| Drinking water source 1 | 0.32±0.003 |
0.57 |
0.877±0.25 |
97.7 |
| Household water sample 1 | 0.775±0.003 |
0.57 |
1.32±0.10 |
95.6 |
| Drinking water source 2 | 0.843±0.003 |
0.57 |
1.42±0.15 |
101.2 |
| Household water sample 2 | 0.725±0.006 |
0.57 |
1.36±0.15 |
111.4 |
| Fe: Iron, Values are means±SD and the significance level, α is equal to 0.05 | ||||
| Table 5: | Recovery test results for water samples | |||
| Metal | Conc. of an unspiked sample (mg L–1) |
Amount added (mg L–1) |
Conc. spiked sample (mg L–1) |
Recovery (%) |
| Ca | 67.1±0.38 |
0.5 |
67.7±0.11 |
108.7 |
| Mg | 27.3±0.18 |
0.5 |
27.8±0.54 |
104.7 |
| Cu | 00.35±0.02 |
0.5 |
00.91±0.02 |
112.7 |
| Mn | 05.42±0.5 |
0.5 |
05.96±0.31 |
107.3 |
| Ca: Calcium, Mg: Magnesium, Cu: Copper, Mn: Manganese, Values are means±SD and the significance level, α is equal to 0.05 | ||||
| Table 6: | Results of selected physicochemical parameters in water source 1, 2 and household 1, 2 | |||
| Parameters | Drinking water source 1 |
RSD (%) |
Household water sample 1 |
RSD (%) |
| Total dissolved solids (mg L–1) | 378.22±7.03 |
1.90 |
379.11±3.30 |
0.9 |
| Electrical conductivity (μS cm–1) | 873.67±3.61 |
0.41 |
874.89±3.59 |
0.41 |
| pH | 7.20±0.04 |
0.60 |
7.16±0.11 |
1.6 |
| Temperature (°C) | 21.74±0.09 |
0.41 |
21.32±0.56 |
2.6 |
| Parameters | Drinking water source 2 |
RSD (%) |
Household water sample 2 |
RSD (%) |
| Total dissolved solids (mg L–1) | 592.78±1.92 |
0.32 |
592.22±2.82 |
0.48 |
| Electrical conductivity (μS cm–1) | 1335.33±12.1 |
0.91 |
1334.22±7.70 |
0.58 |
| pH | 7.40±0.05 |
0.60 |
7.38±0.01 |
0.12 |
| Temperature (°C) | 21.61±0.18 |
0.82 |
21.57±0.64 |
3.00 |
| RSD (%): Relative standard deviation, Values are means±SD and the significance level, α is equal to 0.05 | ||||
pH: The pH is a term used universally to express the intensity of the acid or alkaline condition of a solution. Water mostly used for drinking has a pH recorded to be within the range of 6.5-8.538-41. The pH values of water source 1 were found to be 7.20 and that of water source 2 was 7.4. These pH values were within the acceptable pH range38-41. The comparative levels of the physicochemical parameters such as temperature, pH, electrical conductivity and total dissolved solids between drinking water sources were shown in Table 6.
One-way ANOVA analysis showed that there was a significant difference among the four water samples on the levels of pH, EC and TDS with F (3, 32) = 31.413, p<0.001, F (3, 32) = 10930.750, p<0.001 and F (3, 32) = 7626.759, p<0.001, respectively. Post hoc testing revealed significant differences between pairs of water samples, with pH (7.20±0.04), EC (873.67±3.61) and TDS (378.22±7.03) values of water from drinking water source 1 and with pH (7.16±0.11), EC (874.89±3.59) and TDS (379.11±3.3) values of water from household 1 have less pH, EC and TDS values than water sample from drinking water source 2 pH (7.40±0.05), EC (1335.33±12.1) and TDS (592.78±1.92) and a water sample from household 2 pH (7.38±0.01), EC (1334.22±7.7) and TDS (592.22±2.82). These findings indicated that the drinking water sources are different in their dissolved ions content and the quality of water has not deteriorated between sources and households as it was expected. One-way ANOVA analysis also showed that there was no significant difference among the four water samples in the levels of temperature. The values of the temperature obtained for the two household’s drinking water were not substantially different from those of the sources.
Results of total hardness, total alkalinity and chloride: The values of the physicochemical parameters such as total hardness, total alkalinity and chloride for the selected drinking water sources and household water samples were shown in Table 7.
Total hardness (TH): The study showed the levels of total hardness in all water samples were above the limits of CES-5838 guidelines. As a result, the water from the two sources is considered to be very hard water which mainly causes an aesthetic concern because of the unpleasant taste that a high concentration of calcium and other ions gives to water. Hard water also reduces the ability of soap to produce lather and causes scale formation in pipes and plumbing fixtures. The high levels of hardness in the drinking water sources might be due to naturally occurring processes from the weathering of limestone, sedimentary rock and calcium-bearing minerals, seepage and runoff from soils.
Total alkalinity (TA): Total alkalinity values for drinking water source 2 and household 2 were higher than the drinking water guideline38, but total alkalinity values for drinking water source 1 and household 1 were below the drinking water guideline38. The increased level of total alkalinity observed in drinking water sources 2 might be due to the elevated levels of dissolved carbonate and bicarbonate from weathering of calcium/magnesium carbonate or bicarbonate or carbonate species such as limestone and dolomite, which is likely to increase the carbonate (the alkalinity).
Relationship between alkalinity and hardness: As the total hardness includes both temporary and permanent hardness caused by the calcium and magnesium, total hardness is always greater than total alkalinity which is caused by metals such as calcium and magnesium combined with a form of alkalinity that is attributed to compounds such as carbonate, bicarbonate, hydroxide, etc. In other words, the counter-ions associated with the bicarbonate and carbonate fraction of alkalinity are the principal cations responsible for hardness (usually Ca2+ and Mg2+) as shown in Table 7. As a result, the carbonate fraction of hardness (expressed as CaCO3 equivalents) is chemically equivalent to the bicarbonates of alkalinity present in water42 in areas where the water interacts with limestone43. A hardness value that is greater than the alkalinity represents non-carbonate hardness (permanent hardness).
Chloride: The chloride values were found to be 86.3 mg L–1 and 150.3 mg L–1 for drinking water source 1 and source 2, respectively. These values were below the maximum limit permitted by ESA38 drinking water specifications.
One-way ANOVA analysis showed that there was a significant difference among the four water samples on the levels of total alkalinity, total hardness and chloride with F (3, 8) = 219.033, p<0.001, F (3, 8) = 2101.877, p<0.001 and F (3, 8) = 481.577, p<0.001, respectively. Post hoc testing revealed significant differences between pairs of water samples, with total alkalinity (163.33±2.9) and chloride (86.31±4.2) values of water from drinking water source 1 and with total alkalinity (166.67±7.6) and chloride (82.64±0.58) values of water from household 1 have slightly less than total alkalinity (231.67±2.9) and chloride (150.29±2.5) values from drinking water source 2 and total alkalinity (233.33±2.9) and chloride (151.95±3.6) values for water sample from household 2. These findings indicated that the drinking water sources are different in their content of dissolved ions and chloride and the quality of water has not deteriorated between sources and households as it was expected. However, One-way ANOVA analysis also showed that there was a significant difference among the four water samples on the levels of total hardness.
| Table 7: | Results of total hardness, total alkalinity and chloride in water source 1, 2 and household 1, 2 | |||
| Parameters | Drinking water source 1 |
RSD (%) |
Household water sample 1 |
RSD (%) |
| Total hardness (mg CaCO3 L–1) | 444.54±3.5 |
0.8 |
471.36±4.10 |
0.9 |
| Total alkalinity (mg CaCO3 L–1) | 163.33±2.9 |
1.8 |
166.67±7.60 |
4.6 |
| Chloride (mg L–1) | 086.31±4.2 |
4.8 |
082.64±0.58 |
0.7 |
| Parameters | Drinking water source 2 |
RSD (%) |
Household water sample 2 |
RSD (%) |
| Total hardness (mg CaCO3/L) | 606.17±2.2 |
0.4 |
591.75±2.4 |
0.4 |
| Total alkalinity (mg CaCO3/L) | 231.67±2.9 |
1.3 |
233.33±2.9 |
1.2 |
| Chloride (mg L–1) | 150.29±2.5 |
1.7 |
151.95±3.6 |
2.4 |
| RSD (%): Relative standard deviation, Values are means±SD and the significance level, α is equal to 0.05 | ||||
| Table 8: | Results of the concentration of Ca, Mg, Cu and Mn, in water samples from source 1, 2 and household1, 2 | |||
| Metals | Drinking water source 1 |
RSD (%) |
Household water sample 1 |
RSD (%) |
| Ca | 66.71±0.75 |
1.2 |
66.58±1.60 |
2.6 |
| Mg | 26.40±0.92 |
2.9 |
27.06±1.40 |
4.5 |
| Cu | 0.34±0.02 |
3.7 |
0.36±0.03 |
6.1 |
| Mn | 5.5±0.310 |
1.7 |
5.5±0.280 |
0.8 |
| Metals | Drinking water source 2 |
RSD (%) |
Household water sample 2 |
RSD (%) |
| Ca | 119.01±5.00 |
4.6 |
122.42±6.30 |
3.8 |
| Mg | 74.84±3.50 |
4.8 |
74.72±2.80 |
2.2 |
| Cu | 1.06±0.06 |
2.1 |
1.04±0.02 |
1.6 |
| Mn | 5.31±0.25 |
2 |
5.6±0.340 |
2.2 |
| RSD (%): Relative standard deviation, Ca: Calcium, Mg: Magnesium, Cu: Copper, Mn: Manganese, Values are means±SD and the significance level, α is equal to 0.05 | ||||
Results of the concentration of Ca, Mg, Cu, Mn, Co, Cr, Ni and Pb: The concentration of Co, Cr, Ni and Pb in the water samples analyzed was below the detection limit. However, the results of the concentration of the selected metals such as Ca, Mg, Cu and Mn in drinking water sources and Household water samples were shown in Table 8.
Calcium and magnesium: The calcium ion concentration at drinking water source 1 was 66.7 and 119.0 mg L–1 at drinking water source 2. The magnesium ion content was 26.4 mg L–1 at drinking water source 1 and 78.8 mg L–1 at drinking water source 2. The study showed that the concentration of calcium and magnesium was found at elevated levels38 for water samples taken from source 2. However, the concentration of calcium and magnesium in the water sample taken from source 1 was found within the permissible level of the Ethiopian Standard for Drinking Water Specification38. The drinking water samples analysed from source 2 have a high concentration of calcium in comparison to Mg levels and this might be attributed to a geological source containing soil minerals rich in calcium and the depth of the water in contact with the rock surface aquifer.
Copper: The concentrations of copper in all drinking water samples were found to be below the recommended maximum levels of the Ethiopian Standard for Drinking Water Specification38. However, the concentration of copper found in drinking water source 2 was higher than in drinking water source 1. This might be due to the entry of copper into drinking water as it passes through plumbing systems with copper parts. In other words, copper often occurs in drinking water as a result of corrosion from the plumbing system.
Manganese: The study showed all water samples contained elevated levels of manganese as compared to Ethiopian Standard for Drinking Water Specification38 guideline. The high concentrations of manganese might be due to low oxygen conditions in groundwater or from anthropogenic sources such as municipal wastewater discharges, sewage sludge, mining and mineral processing (particularly nickel), emissions from alloy, steel and iron production and the combustion of fossil fuels.
One-way ANOVA analysis showed that there was a significant difference among the four water samples on the concentration of calcium, F (3, 32) = 525.661, p<0.001, magnesium, F (3, 32) = 1200.486, p<0.001 and copper, F (3, 32) = 1056.055, p<0.001. Post hoc testing revealed calcium, magnesium and copper concentrations in water samples obtained from source 1 and household 1 were not significantly different. Similarly, calcium, magnesium and copper concentration were not significantly different between source 2 (0.82±0.04) and household 2 (0.792±0.06). However, the concentrations of calcium, magnesium and copper were significantly different between Water source 1 and source 2. In addition, a One-way ANOVA analysis showed that there was no significant difference in the concentration of manganese among the four water samples. Thus, these findings indicated that the two drinking water sources were different in the concentration of calcium, magnesium and copper. This might be due to the weathering of carbonate species such as limestone, dolomite and copper-containing minerals that might increase their concentration.
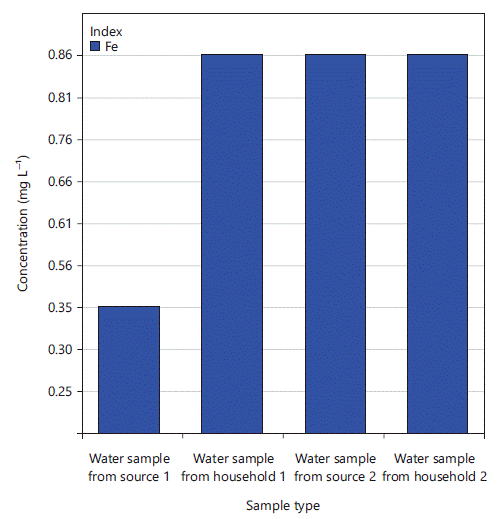
Result of the determination of Fe: The results of iron determination in the selected drinking water samples are shown in Table 9 and Fig. 1. The study revealed that all water samples analyzed contained iron at concentrations above the guideline set by the Ethiopian Standard for Drinking Water Specification38 except water collected from source 1. This might be due to naturally occurring processes, for example from weathering of iron-bearing minerals and rocks or the dissolution of ferrous boreholes and hand pump components. Industrial effluent, acid-mine drainage, sewage and landfill leachate may also contribute to high levels of iron in the local groundwater. The high levels of a water sample taken from household 1 as compared to source 1 may be due to previous iron deposits within pipes and breaking off as rust flakes in water that give high levels of iron in the household water samples. Thus, the water from source 2 should be treated by adding chlorine and ozone or by adding chemicals that cause the metals to form a solid that will settle or be filtered out or used other treatment methods.
One-way ANOVA analysis showed that there was a significant difference among the four water samples in the concentration of iron, F (3, 32) = 123.247, p<0.001. Post hoc testing revealed iron concentration in the water sample obtained from source 1 (0.292±0.03) was substantially different and smaller than the water sample obtained from household 1 (0.697±0.07), source 2 (0.82±0.04) and household 2 (0.792±0.06). In addition, One-way ANOVA analysis showed that there was no significant difference in the concentration of iron between water samples obtained from source 2 and household 2. These findings indicated that the two drinking water sources were different in the concentration of iron and the iron concentration changes very much between source 1 and household 1. This might be due to the rusting of old iron pipe distribution networks that contribute to the increased level of iron in the water sample taken from household 1. However, the concentration of iron was not substantially different between source 2 and household 2 due to the recently constructed water reservoirs and distribution pipes.
Comparison of the levels of the selected metals in drinking water sources investigated with literature values: A summary of the results of the average metal content of the studied water sources in Yabelo Town, Borana Zone, Oromia Regional State Ethiopia and other studies is shown in Table 10. The table also includes national and international recommended limits for selected metals analyzed in drinking water samples.
|
| Table 9: | Results of the concentration of Fe in the selected water samples | |||
| Sample type | Fe concentration (mg L–1) |
Fe concentration (mg L–1) |
RSD (%) |
| Drinking water source 1 | 0.32±0.003 |
0.292±0.03 |
8.8 |
0.286±0.003 |
|||
0.27±0.003 |
|||
| Household water sample 1 | 0.775±0.003 |
0.697±0.07 |
10.1 |
0.674±0.016 |
|||
0.640±0.0029 |
|||
| Drinking water source 2 | 0.843±0.003 |
0.82±0.04 |
4.7 |
0.843±0.003 |
|||
0.775±0.003 |
|||
| Household water sample 2 | 0.725±0.006 |
0.792±0.06 |
7.4 |
0.826±0.0029 |
|||
0.826±0.0029 |
|||
| RSD (%): Relative standard deviation, Values are means±SD and the significance level, α is equal to 0.05 | |||
| Table 10: | Average elemental composition in mg L–1 of water sources from Yabelo Town, Oromia Regional State, Ethiopia | |||
| Metals (mg L–1) |
Source 1 |
Source 2 |
Kasozi et al.44 |
Duressa et al.19 |
Abegaz et al.11 |
CES-58 |
WHO5 |
Andrianou et al.40 |
Shajedul41 |
Health Canada |
| Ca | 66.71 |
119.01 |
- |
- |
- |
75 |
- |
- |
- |
- |
| Mg | 26.4 |
74.84 |
- |
- |
- |
50 |
- |
- |
- |
- |
| Cu | 0.34 |
1.06 |
0.0158 |
- |
- |
2.0 |
2.0 |
2.0 |
1.00 |
1.00 |
| Mn | 5.5 |
5.31 |
- |
1.0 |
0.21 |
0.5 |
0.4 |
0.05 |
0.05 |
<0.02 |
| Fe | 0.292 |
0.82 |
0.512 |
1.19 |
0.51 |
0.3 |
0.3 |
0.2 |
0.3 |
<0.30 |
| CES-58: Compulsory Ethiopian Standard for Drinking Water Specifications, WHO: World Health Organization, EU: European Union, US-EPA: United States Environmental Protection Agency | ||||||||||
Copper: Copper in the water sources sample from Yabelo Town was found to be 0.34 and 1.06 mg L–1 for source 1 and source 2, respectively which is higher than the copper concentration reported in the borehole drinking water source from the Bushenyi District of South-Western Uganda44. The concentration of copper was almost within the limit of 2.0 mg L–1 permitted in drinking water by national and international organizations such as ESA, WHO and the EU38-40.
Manganese: Manganese in the water sources sample from Yabelo Town was found to be 5.5 and 5.3 mg L–1 for source 1 and source 2, respectively is significantly higher than the manganese concentration reported in the water source from Nekemte, Oromia, Ethiopia11 and water source (protected well) from Guto Gida District, Oromia, Ethiopia11. The concentration of manganese found in the water sources exceeded the limit permitted by ESA, WHO, EU, US-EPA and Health Canada38-41. The concentration of manganese in drinking water sources at elevated levels might be due to the natural leaching processes of naturally occurring groundwater minerals, corroded pipes and due to pollution sources. Sources of pollution rich in organic matter (e.g., runoff from landfills, compost, brush or silage piles, or chemicals such as gasoline) can add to the background level by increasing manganese release from soil or bedrock into groundwater. Typically, manganese concentrations from natural processes are low but can range up to 1.5 mg L–1 or higher. Therefore, at concentrations greater than 5.0 mg L–1, manganese may cause a noticeable aesthetic problem that leads to colour, taste and odour problems in water and subsequently potential health effects. Thus, the water from the sources should be treated using suitable methods45 to remove or reduce the manganese before it is distributed to the community for domestic purposes.
Iron: The level of iron in drinking water source 1 was 0.292 mg L–1 which is lower than the values of iron reported by researchers11,19,44. However, the level of iron in drinking water source 2 was 0.82 mg L–1, which is higher than the values of iron reported by researchers11,44 and lower than the value of iron reported by the researcher19. The average value of iron in drinking water source 1 was nearly equal to or below the maximum allowable iron concentration recommended by national and international organizations38-41. However, the concentration of iron found in drinking water source 2 was considerably above the limit permitted by national and international organizations38-41. The increase in iron concentration in drinking water sources might be due to iron pipe corrosion, water percolating through soil and rock that can dissolve minerals containing iron, the depth of the water and the leaching of iron from the bedrock wells that are in contact with water (aquifers). Although the concentration of iron in drinking water sources does not pose a health risk, its effect is noticeable through the staining of clothes and household fixtures such as clothes washers, dishwashers and bathtubs. Iron can also impart a metallic taste to the water and facilitate the accelerated deterioration of pipes, water heaters and home heating systems. Thus, the water from the sources should be treated using suitable methods45,46 to remove or reduce iron before the water is distributed to the community for domestic purposes.
The study was limited to determining selected physicochemical parameters from two water sources and household tap water in Yabelo Town, Borena Zone, Oromia Regional State, Ethiopia. However, drawing a strong conclusion needs to conduct research on many water sources and household tap water samples taken for a certain period. Nonetheless, the results of the present study indicated that the levels of some of the selected water quality parameters were found at elevated levels for all water samples analyzed. Thus, a continuous surveillance and monitoring system of water sources should be in place to ensure the provision of safe and good-quality drinking water for the community. Furthermore, the water supplied to the community through the water distribution system should be treated before distributing to the community to ensure a safe and adequate water supply for the population which has far-reaching effects on health, productivity and quality of life.
CONCLUSION
The results of the present study indicated that the levels of TDS, pH, chloride and copper were found to be below the guideline set by Ethiopian Standard for Drinking Water Specification for all water samples. Furthermore, the concentration of Co, Cr, Ni and Pb was below the detection limit. However, the levels of total hardness and manganese were found to exceed the limit. In addition, the levels of total alkalinity, calcium, magnesium and iron found in drinking water source 2 were above the limit. The result suggested that the drinking water provided to Yabelo Town Community is potable and safe for consumption and doesn’t pose a health risk despite the levels of manganese and iron found at elevated levels. This is because these metals cause aesthetic problems that affect all potential uses of the water (e.g. stains on fixtures or laundry) only and in many cases, these are removed from all water entering the home using Point-of-entry (POE) treatment devices or other treatment methods.
SIGNIFICANCE STATEMENT
The purpose of the research was to assess the levels of selected physicochemical parameters in drinking water sources and tap water supplied to the community in Yabelo Town and consequently assess whether the quality of water delivered to the community by water utilities meets national and international guidelines for drinking water quality. The result suggested that the drinking water provided to the Yabelo Town Community is potable and safe for consumption and doesn’t pose a health risk despite the levels of manganese and iron found at elevated levels.
REFERENCES
- Bos, R., R. Virginia, P. Gérard, J.R. Michael, L. Carolina, M. Neil and A. David, 2016. Manual on the Human Rights to Safe Drinking Water and Sanitation for Practitioners IWA Publishing, London, England, ISBN: 9781780407432, Pages: 110.
- Salehi, M., 2022. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int., 158.
- Mohsin, M., S. Safdar, F. Asghar and F. Jamal, 2013. Assessment of drinking water quality and its impact on residents health in Bahawalpur city. Int. J. Humanit. Social Sci., 3: 114-128.
- Obeta, M.C. and C.F. Nwankwo, 2015. Factors responsible for rural residential water supply shortage in Southeastern Nigeria. J. Environ. Geogr., 8: 21-32.
- WHO/UNICEF, 2014. Progress on Drinking Water and Sanitation: 2014 Update. World Health Organization, ISBN: 9241507241, Pages: 67.
- Amenu, K., D. Shitu and M. Abera, 2016. Microbial contamination of water intended for milk container washing in smallholder dairy farming and milk retailing houses in Southern Ethiopia. SpringerPlus, 5.
- Desye, B., B. Belete, Z.A. Gebrezgi and T.T. Reda, 2021. Efficiency of treatment plant and drinking water quality assessment from source to household, Gondar City, Northwest Ethiopia. J. Environ. Public Health, 2021.
- Lewoyehu, M., 2021. Evaluation of drinking water quality in rural area of Amhara Region, Ethiopia: The case of Mecha District. J. Chem., 2021.
- Tsega, N., S. Sahile, M. Kibret and B. Abera, 2013. Bacteriological and physico-chemical quality of drinking water sources in a rural community of Ethiopia. Afr. Health Sci., 13: 1156-1161.
- Simelane, M.S., M.C. Shongwe, K. Vermaak and E. Zwane, 2020. Determinants of households’ access to improved drinking water sources: A secondary analysis of eswatini 2010 and 2014 multiple indicator cluster surveys. Adv. Public Health, 2020.
- Abegaz, M.T. and M.J. Midekssa, 2021. Quality and safety of rural community drinking water sources in Guto Gida District, Oromia, Ethiopia. J. Environ. Public Health, 2021.
- Sitotaw, B., E. Melkie and D. Temesgen, 2021. Bacteriological and physicochemical quality of drinking water in Wegeda Town, Northwest Ethiopia. J. Environ. Public Health, 2021.
- Mekonnen, G.K., B. Mengistie, G. Sahilu, W. Mulat and H. Kloos, 2019. Determinants of microbiological quality of drinking water in refugee camps and host communities in Gambella Region, Ethiopia. J. Water Sanit. Hyg. Dev., 9: 671-682.
- Kumar, P., F. Arshad, S.K. Shaheen, A. Nadeem, Z. Islam and M.Y. Essar, 2022. Water sanitation in Karachi and its impact on health. Ann. Med. Surg., 77.
- Kibret, F.D. and F.D. Tulu, 2014. Challenges of potable water supply system in rural Ethiopia: The case of Gonji Kolela Woreda, West Gojjam Zone, Ethiopia. Nat. Resour. Conserv., 2: 59-69.
- Berhanu, A. and D. Hailu, 2015. Bacteriological and physicochemical quality of drinking water sources and household water handling practice among rural communities of Bona District, Sidama Zone-Zouthern, Ethiopia. Sci. J. Public Health, 3: 782-789.
- Ali, H., K. Bacha and T. Ketema, 2011. Bacteriological Quality and antimicrobial susceptibility of some isolates of Well Water used for drinking in Jimma Town, Southwest Ethiopia. Ethiop. J. Educ. Sci., 6: 95-108.
- Wolde, A.M., K. Jemal, G.M. Woldearegay and K.D. Tullu, 2020. Quality and safety of municipal drinking water in Addis Ababa City, Ethiopia. Environ. Health Prev. Med., 25.
- Duressa, G., F. Assefa and M. Jida, 2019. Assessment of bacteriological and physicochemical quality of drinking water from source to household tap connection in Nekemte, Oromia, Ethiopia. J. Environ. Public Health, 2019.
- WHO, 2019. Water, Sanitation, Hygiene and Health: A Primer for Health Professionals. World Health Organization, Geneva, Switzerland, Pages: 36.
- UNDP, 2006. Human Development Report 2006: Beyond Scarcity: Power, Poverty and the Global Water Crisis United Nations Development Programme, New York, ISBN: 0230500587, Pages: 422.
- Sila, O.N., 2019. Physico-chemical and bacteriological quality of water sources in rural settings, a case study of Kenya, Africa. Sci. Afr., 2: e00018.
- Bulta, A.L., G.A.W. Micheal, 2019. Evaluation of the efficiency of ceramic filters for water treatment in Kambata Tabaro Zone, Southern Ethiopia. Environ. Syst. Res., 8.
- WHO, 2004. Guidelines for Drinking-water Quality, Volume 1. World Health Organization, Geneva, Switzerland, ISBN:92-4-154638-7, Pages: 366.
- UNICEF and WHO, 2015. Progress on Sanitation and Drinking Water : 2015 Update and MDG Assessment World Health Organization, Geneva, Switzerland, ISBN: 9789241509145, Pages: 80.
- Dawes, J.H.P., 2022. SDG interlinkage networks: Analysis, robustness, sensitivities, and hierarchies. World Dev., 149.
- Rahmanian, N., S.H.B. Ali, M. Homayoonfard, N.J. Ali, M. Rehan, Y. Sadef and A.S. Nizami, 2015. Analysis of physiochemical parameters to evaluate the drinking water quality in the State of Perak, Malaysia. J. Chem., 2015.
- WHO, 2011. Guidelines for Drinking-Water Quality, 4th Edition. World Health Organization, Geneva, Switzerland, ISBN: 9789241548151, Pages: 564.
- Ushie, F.A. and P.A. Amadi, 2008. Chemical characteristics of ground water from parts of the basement complex of Oban massif & Obudu plateau, SE Nigeria. Sci. Afr., 7.
- Amare, A., B. Simane, J. Nyangaga, A. Defisa, D. Hamza and B. Gurmessa, 2019. Index-based livestock insurance to manage climate risks in Borena zone of southern Oromia, Ethiopia. Clim. Risk Manage., 25.
- Worku, M.A., G.L. Feyisa and K.T. Beketie, 2022. Climate trend analysis for a semi-arid Borana Zone in Southern Ethiopia during 1981-2018. Environ. Syst. Res., 11.
- Larson, T.E. and L. Henley, 1955. Determination of low alkalinity or acidity in water. Anal. Chem., 27: 851-852.
- Thomas, J.F.J. and J.J. Lynch, 1960. Determination of carbonate alkalinity in natural waters. J. Am. Water Works Assoc., 52: 259-268.
- Ojobo, K.C. and P. Adowei, 2021. Evaluation of physicochemical characteristics and carbonate equilibria system of the New Calabar River, Choba, Rivers State, Nigeria. J. Appl. Sci. Environ. Manage., 25: 1841-1847.
- Akaho, A.A., G.B. Tikeri and A.O. David, 2022. Physicochemical analysis of potable water in Baham Community, Western Region of Cameroon. J. Appl. Sci. Environ. Manage., 26: 1203-1209.
- Bower, N.W., 1992. Principles of instrumental analysis. 4th edition (Skoog, D.A.; Leary, J.J.). J. Chem. Educ., 69.
- Ripp, J., 1996. Analytical Detection Limit Guidance & Laboratory Guide for Determining Method Detection Limits. Wisconsin Department of Natural Resources, Laboratory Certification Program, Madison WI, Wisconsin, Pages: 24.
- Gintamo, B., M.A. Khan, H. Gulilat, R.K. Shukla and Z. Mekonnen, 2022. Determination of the physicochemical quality of groundwater and its potential health risk for drinking in Oromia, Ethiopia. Environ. Health Insights, 16.
- WHO, 2008. Guidelines for Drinking-Water Quality, 3rd edition: 2nd Addendum to Volume 1. 3rd Edn., World Health Organization, Geneva, Switzerland, ISBN: 9789241547604, Pages: 92.
- Andrianou, X.D., C. van der Lek, P. Charisiadis, S. Ioannou and K.N. Fotopoulou et al., 2019. Application of the urban exposome framework using drinking water and quality of life indicators: A proof-of-concept study in Limassol, Cyprus. PeerJ, 7.
- Shajedul, M.S., 2022. Status of groundwater aquifers, water quality, sources of contamination, and future challenges in Bangladesh: A comprehensive review. J. Appl. Sci. Environ. Manage., 26: 1327-1342.
- Burton, G.A. and R. Pitt, 2001. Stormwater Effects Handbook: A Toolbox for Watershed Managers, Scientists, and Engineers. 1st Edn., CRC Press, Boca Raton, ISBN: 9780429123269, Pages: 928.
- Timmons, M.B., 2002. Recirculating Aquaculture Systems. 2nd Edn., Cayuga Aqua Ventures, New York, ISBN: 0971264619, Pages: 769.
- Kasozi, K.I., S. Namubiru, R. Kamugisha, E.D. Eze and D.S. Tayebwa et al., 2019. Safety of drinking water from primary water sources and implications for the general public in Uganda. J. Environ. Public Health, 2019.
- Baharudin, F., M.Y.M. Tadza, S.N.M. Imran and J. Jani, 2018. Removal of iron and manganese in groundwater using natural biosorbent. IOP Conf. Ser.: Earth Environ. Sci., 140.
- Ellis, D., C. Bouchard and G. Lantagne, 2000. Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination, 130: 255-264.
How to Cite this paper?
APA-7 Style
Zigde,
H.M., Begna,
B.N. (2023). Physicochemical Characteristics of Drinking Water Sources and Household Tape Water in Yabelo Town. Trends in Applied Sciences Research, 18(1), 131-148. https://doi.org/10.3923/tasr.2023.131.148
ACS Style
Zigde,
H.M.; Begna,
B.N. Physicochemical Characteristics of Drinking Water Sources and Household Tape Water in Yabelo Town. Trends Appl. Sci. Res 2023, 18, 131-148. https://doi.org/10.3923/tasr.2023.131.148
AMA Style
Zigde
HM, Begna
BN. Physicochemical Characteristics of Drinking Water Sources and Household Tape Water in Yabelo Town. Trends in Applied Sciences Research. 2023; 18(1): 131-148. https://doi.org/10.3923/tasr.2023.131.148
Chicago/Turabian Style
Zigde, Haile, Melaku, and Biru Negash Begna.
2023. "Physicochemical Characteristics of Drinking Water Sources and Household Tape Water in Yabelo Town" Trends in Applied Sciences Research 18, no. 1: 131-148. https://doi.org/10.3923/tasr.2023.131.148

This work is licensed under a Creative Commons Attribution 4.0 International License.



