Morphorgenic Effect of UV-Light on Two Abelmoschus esculentus (L.) Moench. (Okra) Accessions
| Received 19 Jul, 2024 |
Accepted 30 Oct, 2024 |
Published 31 Oct, 2024 |
Background and Objective: Okra (Abelmoschus esculentus)is an annual vegetable crop belonging to the family Malvaceae; with several cultivated species of economic importance, such as Abelmoschus esculentus and Abelmoschus caillei. This study aimed to identify the interplay between Ultra-Violet C and the morphogenic characters of two okra accessions: Green stem accession (GSA) and red stem accession (RSA). Materials and Methods: Seed exposure to UV-C was conducted at various time intervals: 40, 80, 120 and controls, for both accessions. Data were collected on a weekly basis and analyzed using the Statistical Package for Social Scientists (SPSS) Version 2022, while the level of significance was determined by Tukey’s HDS test at a 0.05% confidence level. Results: No significant UV-C effect on the germination of GSA and RSA seeds, including control. The UV-C did not significantly (p>0.05) affect the growth pattern of both test varieties from weeks 1-10. Microscopic examination of pollen morphology revealed bright-pale yellow, trigonal-irregular-shaped grains tapered at the end, with RSA 120 having the highest pollen size (29.1 μm). The highest leaf number was recorded at GSA80 (16.33±9.24) and RSA80 (16.87±3.84) at week 10. Petiole length was highest at GSA120 (24.67±2.60), followed by RSA120 (22.33±0.67). Capsule number and yield increased with an increase in UV-C doses from 40 to 120, with the lowest recorded among the controls. Conclusion: The UV-C irradiation at 120 min or more should be used for high-yielding okra varieties. This will promote both nutritional and nutraceutical qualities of the test crop and help in the fight against nutrient-deficiency diseases; especially in rural communities.
| Copyright © 2024 Ehumadu et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Okra (Abelmoschus esculentus) is one of the most important vegetable crops with tremendous nutritional values1,2. The origin of okra is traceable to the old world and is generally grown in tropical regions under hot weather conditions3. Okra is grown extensively in Nigeria and other African countries for its numerous nutritional and medicinal benefits as well as income generation1,4. To meet the demand for nutritionally balanced food for the world’s increasing population and relieve the intense pressure on the valuable food supply chain, food crops must be diversified through appropriate scientific protocols that will meet human nutritional needs and create a sustainable environment5. Ultraviolet (UV) radiation has been identified as an important type of mutagen because it is easily absorbed by the genetic materials of living cells to induce mutation6. Ultra-violet radiation is divided into three groups based on their wavelengths. The first category is UV-A, which has a wave length between 315 and 400 nm and constitutes 95% of UV radiation that penetrates the ozone layer. Gentili et al.7 noted that UV-B (280-315 nm) constitutes 5% of UV radiation and it’s also called burning radiation, due to the formation of photoproducts that damage the DNA while UV-C (200-280 nm) has a short wavelength, which is completely absorbed by the ozone layer. It is characterized by its high ability to kill microorganisms and is therefore used for sterilization purposes8.
Exposure to UV radiation for a long time may lead to the formation of free radicals and thus cause DNA damage4. The absorption of UV rays by plant cells can lead to metabolic, biochemical and morphological alterations, as well as changes in the genetic materials9 to produce genetically distinct cultivars of the same crop. The numerous cultivars vary in time to maturity, color of leaves, stem length, shape of the fruit and other characteristics10. The morphological characters such as number, dimensions and persistency of the epicalyx segment, form and dimensions of the fruit (including the pedicel) and characteristics of the integuments are unique11. For example, the main okra types are short-and long-duration yielding cultivars, which have numerous accessions within them12. Despite numerous efforts by scientists, the available okra varieties are yet to meet local and global demands, such as early maturity, high-yielding potential, mucilage viscosity and climate resilience, among other desired and desirable traits. Therefore, this experiment was designed to investigate the morphogenic effect of UV-C light on two okra accessions.
MATERIALS AND METHODS
Study location: The experiment was conducted in a greenhouse measuring 8×8 ft long and 10 ft high, belonging to the Department of Biology, Federal University of Technology, Owerri, Imo State, Nigeria (5°2333.6876”N and 6°5910.5504”E); from February to July, 2023.
Source of test crops (okra seeds): Dry fruit pods (50 seeds) of the two okra accessions identified as Red Stem Accession (RSA) and Green Stem Accession (GSA) were bought from a rural farmer in Ihiagwa Community Market, Owerri West LGA, Imo State.
Experimental procedure
Seed viability test (SVT): Dry fruit pods of the test okra accessions (RSA and GSA) were manually broken to recover their respective seeds. Prior to the viability test, seeds were first sorted out to select healthy-looking ones, which were labelled accordingly2. The second stage of viability assessment was conducted using the flotation method8. Sorted seeds were later transferred into two separate plastic plates, each containing 15 CL of tap water13 and were allowed to stand for 10 min. Non-floating seeds were recovered, while floating ones were assumed to be non-viable and discarded14.
Experimental design: The experiment was conducted in a Completely Randomized Design (CRD). The 10 viable seeds each of RSA and GSA were put in 4 Petri dishes representing treatment levels (min), including control, marked as RSA40, RSA80, RSA120 and control (RSAC) and GSA40, GSA80, GSA120 and control (GSAC), respectively2.
UV-C irradiation: The UV irradiation was conducted in the general laboratory of the Department of Biology, FUTO, the day after the viability assessment was conducted. The aim was to enhance integument permeability as well as break dormancy for easy penetration of UV-rays8,13,15. The A UV-C germicidal lamp of 280 nm wavelength, fitted in a hood, was deployed for the irradiation. The 10 selected seeds, each of RSA and GSA, spread in each codified Petri dish were placed at a distance of 22 cm from the lamp15. The time of exposure varied at a 40 min interval for all treatment levels, except the controls.
Soil preparation and planting: Two-ridge rows, measuring 2 m (approximately 6.6 ft) each, were prepared after clearing and observance of necessary cultural practices14. The 3 replicate viable seeds per treatment, per accession, were selected from the pool of 10 irradiated seeds and planted 4 cm apart in a planting hole of 5 cm depth and an inter-treatment distance of 15 cm16. Weeding was conducted at weekly intervals prior to the measurement of stipulated vegetative parameters15.
Germination parameters
Determination of germination duration: This was determined by observing the emergence of epicotyls on the day of planting (DOP) and recording accordingly, as reported by Dias et al.17.
Germination percentage: The percentage of seed germination was calculated on the basis of the total number of seeds germinated against the total number of seeds sown as reported by Olawuyi et al.13 and expressed as:
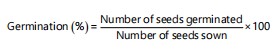 |
Measurement of agronomic characters: The following agronomic characters of the test okra accessions were determined as follows:
| • | Shoot length: With the aid of a calibrated measuring tape, the crop height was determined by the method of Sargheini et al.15. Measurements were taken from the base of each plant stand to the shoot tip. This was done weekly for 10 consecutive weeks. The mean shoot length was calculated in centimeters (cm)18 | |
| • | Leaf number: The leaf number was determined manually by counting the leaves weekly for a cumulative period of 10 weeks. Young or not well-developed leaves were not counted15,17 | |
| • | Leaf area: The leaf area was calculated by using the transparent grid paper method16. The leaf was placed on a grid graph sheet. Boxes covered by the leaf were counted while areas covered by less than half of the leaf were not counted15. The mean leaf area of each treatment and control was calculated and expressed in centimeters squared (cm2)18 | |
| • | Petiole length: This was determined using a transparent ruler calibrated in centimeters. The measurement was taken from the point of attachment on the stem to the leaf base and was done weekly. The mean petiole length was calculated and expressed in centimeters19,20 | |
| • | Inflorescence, emergence and color: The inflorescence emergence was determined by closely monitoring the aerial stalk for young inflorescence development, while inflorescence color was determined in broad daylight after flowers were fully blossomed following eye examination, as reported by Nwangburuka et al.21. Color changes were closely monitored and recorded accordingly22 | |
| • | Pollen morphology: The pollen morphological parameters were determined by gently shaking the flower over a clean surface of a microscopic slide, covered with a cover slip with a drop of glycerin, before being examined and micrographed under a digital research microscope (Celestron LCD Digital Microscope. Warsaw, Poland) at x400 magnification22-24 | |
| • | Capsule yield: Fresh okra capsules were harvested according to their accessions and treatment levels and weighed separately using a Camry LED display digital weighing balance, Zhongshan Jinli, China; according to the method of Olawuyi et al.13 |
Data analysis: Data obtained in the course of this investigation were statistically analyzed using One-way Analysis of Variance (ANOVA) and mean separation was carried out by Tukey’s HSD at p<0.0525.
RESULTS AND DISCUSSION
The results of the morphogenic effect of UV light on two okra accessions were presented below.
Germination percentage: The result of the germination percentage for GSA and RSA below shows that all treatment levels recorded 100% germination, including control (Table 1). This implies that the germination of both okra accessions and their control were not affected by the UV light. Although seed germination was recorded between the 2nd and 4th days after planting (DAP) to reach full epicotyl emergence above the soil, there was variation in the test seed, irradiation pattern and dosage. Sripong et al.26 and Khan et al.27 reported a gradual decrease in pea (Pisum sativum) germination after UV-C irradiation for a period of 30 to 60 min per day for 7 days. They reported germination percentages of 86.00 and 60.0 in the UV treatment doses for T1 and T2, respectively. The reason for the lack of a significant effect of the mutagen on the germination of both okra accessions could be due to a low or non-repeated dosage of UV irradiation on the okra seeds26. They further revealed that seed germination and 50% epicotyl emergence took 1-2 and 2-3 days, respectively, in controls. The reduction in germination percentage of the treated seeds could be attributed to disturbances in the genetically controlled bio-physiological metabolic pathways necessary for seed germination that may include enzyme activity, hormonal imbalances and inhibition of the mitotic process27. It was also documented that the inhibition of DNA synthesis due to the induced mutations by certain mutagens may be the reason for reduced seed germination28,29. The 100% germination recorded in this experiment differs from that of Çavuşoğlu et al.30 in Allium cepa. The observed difference could be due to variations in test plants, irradiation type or dosage31.
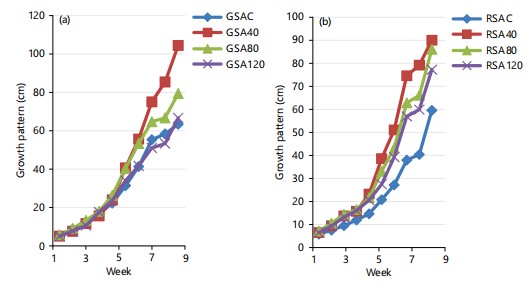
Plant growth for both okra accessions: Results showed that the growth pattern of both GSA and RSA (Fig. 1a-b) at all treatment levels of the experiment was significantly different (p>0.05) from weeks 1-10. However, both okra accessions maintained a regular exponential growth pattern with time for all treatment levels of both accessions, including their controls, except the GSA120 and RSA120, where plant growth was retarded. This result confirmed the earlier studies by Zuk-Golaszewska et al.31, who studied the effects of UV-B radiation on plant growth and development using Avena fatua and Setaria viridis. They reported that increased UV-B irradiation delayed plant growth during the experiment. The differences in mean plant height at 0, 4 and 8 kJ/m2/d UV-B radiation were non-significant and only for 12 kJ/m2/d significant plant height reduction was observed. This further confirmed the result of AL-Jarrah and AL-Qazwini32, who also reported that prolonged exposure to UV-C affects plant height and relative growth rate in some rice cultivars. In this research, both okra accessions showed normal growth in their response to UV light, even though phenotypic changes were observed mostly in their height upon terminating the experiment.
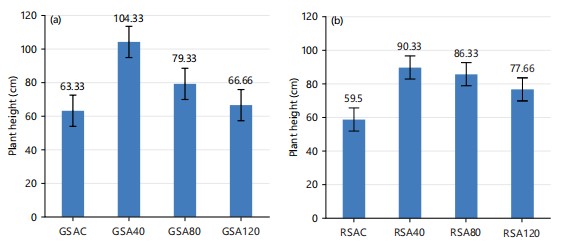
Plant height for both okra accessions: The results of plant height in both okra accessions (GSA and RSA) as shown in Fig. 2a-b below were significantly different (p<0.05) as a result of the effect of the different doses of UV radiation. This was in agreement with the work of Techavuthiporn et al.2, who worked on the mutagenic effects of UV irradiation on growth and agronomic characters in maize cultivars. From their investigation, the effects of UV irradiation on the first-order interaction between weeks after planting (WAP) and treatment were only significant (p<0.05) on plant height. It showed a reduction in height at 10 WAP. This is due to the interval of exposure periods, which was 20 min and the genetic stressors of maize cultivars. However, UV light seems to have induced uniform height in both okra accessions, with GSA and RSA at 40% irradiation having the tallest heights. On the other hand, controls of both test okra varieties were shorter than other treatment levels. The regular variation in the height of both test crops conforms to the report of several workers who maintained that mutagenesis has been widely used as a potent method of enhancing variability for crop improvement32. Induced mutation, using physical mutagens, is one of the ways to generate genetic variation, resulting in the creation of new varieties with better characteristics33. The above results imply that the longer the duration of UV-light irradiation, the shorter the height of both okra accessions34.
| Table 1: | Germination percentage of GSA and RSA okra accessions | |||
| Treatment | Germination (%) |
| GSAC | 100 |
| GSA40 | 100 |
| GSA80 | 100 |
| GSA120 | 100 |
| RSAC | 100 |
| RSA40 | 100 |
| RSA80 | 100 |
| RSA12 | 100 |
|
|
Leaf number: The effect of UV light on the leaf number of okra is a multifaceted phenomenon shaped by genetic, physiological and ecological factors. From the study (Table 2), the leaf numbers of both test accessions are not significantly different from week 1-10. Studies on the effect of UV light on okra leaf number have yielded mixed results. Some studies have reported a decrease in leaf number under UV exposure, indicating a potential inhibitory effect on leaf initiation or expansion5.
Conversely, other studies have documented an increase in leaf number, suggesting a stimulatory effect of UV light on leaf primordial development20. Apparently, this study shows no statistical difference in the leaf number of two okra accessions irradiated at different times. These divergent responses highlight the complexity of UV-leaf interactions and the importance of considering factors such as UV intensity, exposure duration, growth conditions or even exposure distance19. The effect of UV light on okra leaf is likely mediated through physiological mechanisms involving hormone signaling, metabolic pathways and nutrient availability35,36. The intricate interplay between these factors determines whether UV light enhances or suppresses leaf numbers. However, different okra varieties may exhibit varying responses to UV light in terms of leaf number. Some varieties could possess genetic traits that confer UV tolerance and lead to consistent leaf production under UV exposure, while others may be more sensitive26.
The effects of UV light on okra leaf number extend beyond individual plant growth. Leaf number influences resource allocation, carbon assimilation and overall plant performance. Altered leaf development under UV exposure could affect the competitive ability of okra in mixed plant communities. Additionally, herbivore interactions and plant-microbe relationships may be influenced by UV-mediated changes in leaf number. Altered leaf development can impact plant competition dynamics, nutrient cycling and energy flow within ecosystems.
Effect of UV light on the leaf area of GSA and RSA treatments: Results on the total leaf area of the studied okra accessions indicate that UV light had no significant effect on the leaf area of both okra accessions (Table 3).
This study was in conformity with research carried out by Ahandani et al.37 on the effect of Ultraviolet (UV) radiation on the growth of Dracocephalum moldavica, grown in a uniform environment in the culture room and exposed to UV rays (20 min of UV-A daily and UV-C for 10 min) at a 10 day interval for 6 weeks. Indices of leaf area were investigated and results of the comparison of the mean of the studied traits showed that there was a significant difference between treatments, so that the highest amount of leaf area was related to the control. Jarerat et al.33 and Derebe et al.38 reported that the Colocasia esculenta plant at high altitude showed a significant morphological response, especially on the leaf area index (LAI), petiole length and specific leaf area, due to the high level of radiation.
This study shows that leaf area was not affected by UV rays at the dosage levels to which both okra accessions were exposed. This result supported the findings of Darras et al.39, who suggested that the UV-C radiation was powerful enough to give maximum benefits so that plants did not need to expand their leaf area to capture more light radiation. The non-significant effect of UV light on the okra leaf area implies that gibberellins, which promote cell elongation and expansion, were not equally altered in both okra accessions39. The observed insignificant effect of UV irradiation on the studied crops could depend on a number of factors, such as dose, method of irradiation, UV type or even the test plant factor40.
Petiole length: The interplay between UV light and plant petiole length in both okra accessions showed that GSA120 and RSA 40 had the longest mean petiole lengths of 24.50±0.29 and 27.00±2.65, respectively (Table 4). Generally, there was no significant difference in the petiole length of the studied okra accessions compared to their controls. This result is contrary to the report that UV light can significantly affect petiole length in okra plants. It also disagreed with the report of Kamal et al.18, which maintained that UV exposure can trigger shifts in gene expression patterns.
| Table 2: | Effect of UV-C on the Leaf number of GSA and RSA accessions | |||
| Treatment | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 |
| GSAC | 3.00±0.00a | 3.67±0.33a | 6.00±0.58a | 7.00±0.58a | 9.00±0.58a | 8.33±0.33a | 8.67±0.88a | 11.00±2.52a | 12.67±2.67a | 13.33±3.33a |
| GSA40 | 2.67±0.33a | 3.67±0.33a | 6.00±0.58a | 7.67±0.88a | 9.00±1.00a | 9.33±2.40a | 10.00±2.08a | 11.67±2.19a | 12.67±1.76a | 12.67±1.76a |
| GSA80 | 3.00±0.00a | 4.00±0.58a | 6.00±0.58a | 8.67±0.33a | 9.00±1.16a | 9.67±2.03a | 10.67±2.40a | 13.33±3.84a | 12.33±6.33a | 16.33±9.24a |
| GSA120 | 3.00±0.00a | 3.33±0.67a | 6.33±1.16a | 6.33±0.33a | 7.67±0.88a | 9.00±0.58a | 9.67±0.88a | 12.00±1.15a | 13.00±1.00a | 15.00±0.57a |
| RSAC | 1.67±0.88a | 2.33±1.20a | 3.00±1.53a | 3.67±1.86a | 3.33±2.03a | 4.33±2.33a | 5.67±3.18a | 6.33±3.76a | 7.00±4.04a | 7.67±4.63a |
| RSA40 | 3.00±0.00a | 4.00±0.00a | 6.67±0.58a | 8.33±0.33b | 9.33±1.45a | 10.67±2.19a | 11.33±2.03a | 11.67±1.45a | 13.67±1.45a | 13.67±1.33a |
| RSA80 | 3.00±0.00a | 4.33±0.33a | 6.00±0.58a | 9.00±0.58b | 9.33±1.45a | 10.67±1.86a | 13.67±3.18a | 15.33±3.71a | 16.33±3.67a | 16.87±3.84a |
| RSA120 | 3.00±0.00a | 4.33±0.33a | 8.33±0.33a | 8.33±0.33b | 9.33±0.33a | 10.33±0.33a | 11.67±0.67a | 12.33±0.88a | 13.00±1.00a | 13.00±1.00a |
| Means followed by the same alphabet within column are not significantly different by Tukey’s HSD at (p>0.05) and Mean±SE (n = 3) | ||||||||||
| Table 3: | Effects of UV-light on leaf area (cm2) of GSA and RSA accessions | |||
| Treatment | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 |
| GSAC | 6.90±0.00a | 8.52±0.00a | 8.81±0.00a | 9.50±0.00a | 9.00±0.00a | 9.00±0.00a | 10.00±0.00a | 11.00±0.00a | 12.00±0.00a | 13.91±0.00a |
| GSA40 | 5.24±0.00a | 7.96±0.00a | 8.74±0.00a | 9.27±0.00a | 9.00±0.00a | 9.17±0.00a | 10.33±0.00a | 11.00±0.00a | 12.00±0.00a | 13.00±0.00a |
| GSA80 | 6.24±0.00a | 8.75±0.00a | 8.65±0.00a | 8.75±0.00a | 9.59±0.00a | 9.27±0.00a | 10.28±0.00a | 11.00±0.00a | 12.00±0.00a | 13.50±0.00a |
| GSA120 | 6.24±0.00a | 8.51±0.00a | 8.69±0.00a | 8.33±0.00a | 9.21±0.00a | 9.00±0.00a | 10.59±0.00a | 12.14±0.00a | 12.00±0.00a | 13.24±0.00a |
| RSAC | 6.75±0.00a | 3.67±0.33a | 6.00±0.58a | 7.00±0.58a | 9.00±0.58a | 8.33±0.33a | 8.67±0.88a | 11.00±2.52a | 12.67±2.67a | 13.33±3.33a |
| RSA40 | 3.00±0.00a | 3.67±0.33a | 6.00±0.58a | 7.67±0.88a | 9.00±1.00a | 9.33±2.40a | 10.00±2.09a | 11.67±2.19a | 12.67±1.76a | 12.67±1.76a |
| RSA80 | 2.67±0.00a | 4.00±0.58a | 6.00±0.58a | 8.67±0.33a | 9.00±1.16a | 9.67±2.03a | 10.67±2.40a | 13.33±3.84a | 12.33±6.33a | 16.33±9.24a |
| RSA120 | 3.00±0.00a | 3.33±0.68a | 6.33±0.68a | 6.33±0.33a | 7.67±0.33a | 9.00±0.58a | 9.67±0.88a | 12.00±1.16a | 13.00±1.00a | 15.00±0.58a |
| Means followed by the same alphabet within column are not significantly different by Tukey’s HSD at (p>0.05) and Mean±SE (n = 3) | ||||||||||
| Table 4: | Effect of UV light on the Petiole length (cm) of GSA and RSA accessions | |||
| Treatment | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | ||||||||||
| GSAC | 1.50±0.29a | 3.53±0.74a | 4.33±0.17a | 8.27±0.43a | 10.17±4.78a | 11.50±1.32a | 16.67±1 .45a | 18.83±1.42a | 20.67±1.33a | 22.00±1.15a | ||||||||||
| GSA40 | 0.83±0.167a | 2.00±0.29a | 3.83±0.67a | 9.00±1.04a | 11.17±1.01a | 13.67±2.19a | 15.17±2.68a | 18.17±2.68a | 19.67±2.73a | 20.33±2.85a | ||||||||||
| GSA80 | 1.53±0.27a | 3.00±0.00a | 5.17±0.97a | 8.87±1.44a | 13.00±3.51a | 16.67±4.06a | 20.33±6.17a | 23.00±7.64a | 22.00±11.06a | 23.67±11.92a | ||||||||||
| GSA120 | 1.67±0.33a | 2.83±0.17a | 4.50±0.58a | 9.67±1.09a | 11.17±1.01a | 14.17±2.13a | 18.67±1.86a | 22.17±3.09a | 24.00±3.00a | 24.67±2.60a | ||||||||||
| RSAC | 0.57±0.47a | 1.43±0.74a | 1.67±0.83a | 3.67±2.03a | 10.13±1.31a | 6.50±3.76a | 8.33±4.91a | 10.00±5.77a | 11.33±6.36a | 13.00±7.00a | ||||||||||
| RSA40 | 1.17±0.17a | 2.67±0.44a | 5.50±0.29b | 11.17±0.44b | 14.00±1.44b | 19.67±1.33b | 20.67±0.88a | 18.33±3.67a | 25.33±2.19a | 27.00±2.65a | ||||||||||
| RSA80 | 1.67±0.17a | 3.00±0.00a | 4.50±0.28b | 9.00±1.04ab | 10.67±1.88ab | 14.67±2.96ab | 16.33±3.28a | 19.50±1.26a | 20.33±3.67a | 20.33±3.67a | ||||||||||
| RSA120 | 1.33±0.17a | 2.33±0.17a | 4.33±0.33b | 9.00±0.76ab | 11.33±0.33ab | 15.00±1.00ab | 19.00±1.04a | 10.00±5.77a | 21.67±0.88a | 22.33±0.67a | ||||||||||
| Means followed by the same alphabet within column are not significantly different by Tukey’s HSD at (p>0.05) and Mean±SE (n = 3) | ||||||||||||||||||||
| Table 5: | Inflorescence emergence and colour for both okra accessions | |||
| Treatment | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 |
| GSAC | NI | NI | NI | + | + |
| GSA40 | NI | NI | NI | + | + |
| GSA80 | NI | NI | NI | + | + |
| GSA120 | NI | NI | NI | NI | + |
| RSAC | NI | NI | + | + | + |
| RSA40 | + | + | + | + | + |
| RSA80 | + | + | + | + | + |
| RSA120 | + | + | + | + | + |
| NI: No inflorescence and +: Presence of inflorescence | |||||
A report has it that exposure of okra to UV-B radiation has led to alterations in petiole length20. Studies have further shown both stimulatory and inhibitory effects, depending on factors such as UV dose, exposure duration and growth conditions19. Studies by Dukowic-Schulze et al.41 and Yamaga et al.42 found that UV-B exposure resulted in d ecreased petiole length in okra plants, suggesting that UV-B may have a negative impact on elongation processes. In contrast, a study by Zhang et al.35 observed increased petiole length in response to moderate UV-B exposure, indicating a potential role of UV-B in promoting elongation under certain conditions. As expected, petiole length increased with time (week 1-10), conforming to the general growth rule2,13. The insignificant UV-C effect on the petiole compared to the reports by Darras et al.39 could be due to differences in UV wavelength, time of exposure or distance between the material and radiation source.
Inflorescence emergence and color for both okra accessions: Results from the table below show that the inflorescence of both GSA and RSA produced flowers with bright yellow-colored petals for all treatment levels, including control38. This implies that UV light did not affect flower color in both accessions at all treatment levels, including control. This was contrary to the report by Abdul Kareem et al.8 that UV-C can induce oxidative results and genetic mutations in plants that in turn have strong negative effects on plant morphology, flowering, pollination, transpiration and photosynthesis.
Similarly, Valenta et al.43 observed phenotypic changes in the inflorescence color in Zinnia. They noted that the UV reflectance on flower color could aid pollination by attracting pollinators43,44. The RSAC produced flowers from week 8, while GSAC, together with GSA40, had flowers emerge from week 9 and GSA120 from week 10 (Table 5). This was contrary to the work of Eskins45, who found that flower morphology is adversely affected by elevated UV-C radiation in soybeans. It was further observed that RSA40-120 treatment levels had flowers emerge at week 6, while none were recorded among all the GSAs until week 8. The observed disparity in flower emergence among both okra accessions could be attributed to differences in their genotype20 and not the treatment or treatment levels.
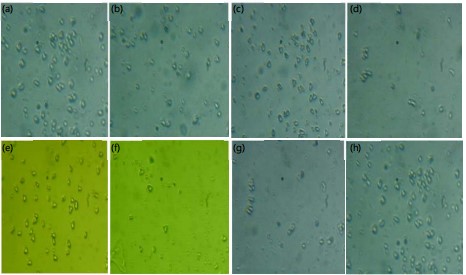
Pollen morphology of GSA and RSA: The results showed that UV light did not significantly affect the color of the pollen grains of both okra accessions; hence, all pollen grains maintained a bright yellow colouration trait, including those of the controls (Fig 3a-h) Similarly, the shape of pollen grains was not significantly affected by the mutagen. All maintained a trigonal-irregular shape with a tapered end. An increase in the UV irradiation dosage from 40 to 120 min observably increased the size of pollen grains in both okra accessions. Several studies have investigated the effect of UV-C radiation on pollen morphology in okra seeds. Although the present study did not take into consideration the viability of pollen grains, it partly agreed with the study by Kumar et al.46, which found that exposure to UV-C radiation led to a decrease in the germination rate of okra pollen grains. This decrease in pollen viability could hitherto be linked to the variation in pollen shape as recorded in this study29. These findings suggested that UV-C radiation can have a significant impact on pollen morphology in okra seeds. A similar observation was made in RSAC-120. Contrary to the work of Alafiatayo et al.47, which maintained that exposure to UV-C radiation led to a decrease in the size and viability of pollen grains in okra plants, the results revealed a significant increase in pollen size from 23.60 μm in GSAC to 27.70 μm in GSA120, while a similar increase between 24.40 and 29.1 μm was recorded in RSAC and RSA120, respectively (Table 6).
|
| Table 6: | Pollen morphology of GSA and RSA accessions | |||
| Treatment | Colour | Shape | Size (μm) |
| GSAC | Bright yellow | Trigonal-irregular | 23.6 |
| GSA40 | Bright yellow | Trigonal-irregular | 27.6 |
| GSA80 | Bright yellow | Trigonal-irregular | 27.4 |
| GSA120 | Bright yellow | Trigonal-irregular | 27.7 |
| RSAC | Bright yellow | Trigonal-irregular | 24.4 |
| RSA40 | Bright yellow | Trigonal-irregular | 28.6 |
| RSA80 | Bright yellow | Trigonal-irregular | 28.8 |
| RSA120 | Bright yellow | Trigonal-irregular | 29.1 |
| Table 7: | Capsule yield (g) of the two okra accessions | |||
| Treatment | Number of fruits per treatment | Total yield (g) |
| GSAC | 18 | 318.84 |
| GSA40 | 27 | 406.2 |
| GSA80 | 29 | 564.84 |
| GSA120 | 32 | 571.65 |
| RSAC | 21 | 147.71 |
| RSA40 | 34 | 600 |
| RSA80 | 38 | 665.41 |
| RSA120 | 44 | 727.64 |
Effect on yield of the two okra accessions: The UV-C treatment affected the capsule number and yield of both okra accessions (Table 7). The lowest and highest yields were recorded in the control and 120 min irradiation doses, a similar result previously reported by Techavuthiporn et al.2. The results clearly showed that an increase in UV irradiation enhanced both capsule number and weight, which was recorded as yield. This result confirmed the earlier studies by Yoshikawa et al.48, who worked on the ‘Improvement of Plant Growth and Yield’ and concluded that UV-C treatment at lower dosages enhances the yield of plants. The amount of yield produced by the control of the two accessions was significantly low, indicating that the UV-C treatment boosted the yield of the okra accessions. By treating soybean seeds at various levels of UV-C, a positive hormetic response was triggered that resulted in enhanced yields of harvested seeds48. The increase in capsule number and yield of both okra accessions, with 120 min giving the highest, suggests that the optimum UV-C dosage was yet to be realized in this present study. This further suggests that higher doses, above 120 min, could give a better yield response in both okra accessions19.
CONCLUSION
Ultraviolet light (UV-light) is considered one of the most important types of mutagens, due to its ability to penetrate genetic materials of living cells thereby causing mutation. This study aims to improve the agronomic qualities of two okra accessions using UV-irradiation. It was observed that the physical mutagen significantly changed the growth and yield patterns of both okra accessions with increase in irradiation time doses. The resultant variant (M1) of both accessions at the 120 min irradiation dose should be studied at the second mutant generation (M2) level prior to their adoption. Therefore, the study will set a new record in the breeding program of okra, foster collaboration among researchers and open a new vista for future studies in the agricultural sector.
SIGNIFICANCE STATEMENT
This study focuses on the effect of UV-C on two okra accessions. The study aimed to improve capsule quality and enhance yield. The treatment had no significant effect on the growth pattern, pollen morphology and other studied vegetative parameters, but showed a strong increased effect on their capsule number and yield. The study indicates that pretreatment of okra seeds using UV-C has a strong potential to boost production and supply of the vegetable crop as well as stabilize recurrent price dynamics due to seasonal availability. This will no doubt enhance food security and create more jobs along the value chain. Lastly, the study will set a new record in the breeding program of okra, foster collaboration among researchers and open a new vista for future studies in the agricultural sector.
REFERENCES
- Neugart, S., M.A. Tobler and P.W. Barnes, 2021. The function of flavonoids in the diurnal rhythm under rapidly changing UV conditions-A model study on okra. Plants, 10.
- Techavuthiporn, C., A. Jarerat, C. Singhkai and H. Nimitkeatkai, 2024. Postharvest UV-C treatment affects bioactive compounds and maintains quality of okra (Abelmoschus esculentus L.) during storage. Hortic. J., 93: 15-22.
- Ndunguru, J. and A.C. Rajabu, 2004. Effect of okra mosaic virus disease on the above-ground morphological yield components of okra in Tanzania. Sci. Hortic., 99: 225-235.
- Georgiadis, N., C. Ritzoulis, G. Sioura, P. Kornezou, C. Vasiliadou and C. Tsioptsias, 2011. Contribution of okra extracts to the stability and rheology of oil-in-water emulsions. Food Hydrocolloids, 25: 991-999.
- Dong, W., L. Shi, S. Li, F. Xu, Z. Yang and S. Cao, 2023. Hydrogen-rich water delays fruit softening and prolongs shelf life of postharvest okras. Food Chem., 399.
- Hideg, É., M.A.K. Jansen and Å. Strid, 2013. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci., 18: 107-115.
- Gentili, P.L., A.L. Rightler, B.M. Heron and C.D. Gabbutt, 2016. Extending human perception of electromagnetic radiation to the UV region through biologically inspired photochromic fuzzy logic (BIPFUL) systems. Chem. Commun., 52: 1474-1477.
- Abdul Kareem, K.A., T.J. Olobatoke, A.A. Abdul Rahaman and O.T. Mustapha, 2019. Mutagenic effects of ultraviolet (UV-C) irradiation on the anatomy of three species of capsicum. Bangladesh J. Sci. Ind. Res., 54: 111-116.
- Carvalho, S.D. and J.A. Castillo, 2018. Influence of light on plant-phyllosphere interaction. Front. Plant Sci. 9.
- Karimi, S., V. Tavallali, M. Rahemi, A.A. Rostami and M. Vaezpour, 2009. Estimation of leaf growth on the basis of measurements of leaf lengths and widths, choosing pistachio seedlings as model. Aust. J. Basic Appl. Sci., 3: 1070-1075.
- Verdaguer, D., M.A.K. Jansen, L. Llorens, L.O. Morales and S. Neugart, 2017. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci., 255: 72-81.
- Lengsfeld, C., F. Titgemeyer, G. Faller and A. Hensel, 2004. Glycosylated compounds from okra inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Agric. Food Chem., 52: 1495-1503.
- Olawuyi, O.O.J. O.B. Bello and A.O. Abioye, 2016. Mutagenic effects of ultraviolet radiation on growth and agronomic characters in maize cultivars. Mol. Plant Breed., 7: 1-10.
- Yao, Y., Z. Xuan, Y. Li, Y. He, H. Korpelainen and C. Li, 2006. Effects of ultraviolet-B radiation on crop growth, development, yield and leaf pigment concentration of tartary buckwheat (Fagopyrum tataricum) under field conditions. Eur. J. Agron., 25: 215-222.
- Sarghein, H.S., J. Carapetian and J. Khara, 2008. Effects of UV-radiation on photosynthetic pigments and UV absorbing compounds in Capsicum longum (L.). Int. J. Bot., 4: 486-490.
- Al Mahroos, M., M. Yaar, T.J. Phillips, J. Bhawan and B.A. Gilchrest, 2002. Effect of sunscreen application on UV-induced thymine dimers. Arch Dermatol., 138: 1480-1485.
- Dias, G.B., V.M. Gomes, T.M.S. Moraes, U.P. Zottich and G.R. Rabelo et al., 2013. Characterization of Capsicum species using anatomical and molecular data. Genet. Mol. Res., 12: 6488-6501.
- Kamal, M.I., A.H. Abd-El-Hadi, K.A. Zaied and A.S. Ahmed, 2023. Mutagenic effect of ultraviolet radiation on seeding growth and productivity of summer squash. Ann. Agric. Sci., Moshtohor, 61: 151-166.
- Biever, J.J., D. Brinkman and G. Gardner, 2014. UV-B inhibition of hypocotyl growth in etiolated Arabidopsis thaliana seedlings is a consequence of cell cycle arrest initiated by photodimer accumulation. J. Exp. Bot., 65: 2949-2961.
- Durazzo, A., M. Lucarini, E. Novellino, E.B. Souto, P. Daliu and A. Santini, 2019. Abelmoschus esculentus (L.): Bioactive components’ beneficial properties-focused on antidiabetic role-for sustainable health applications. Molecules, 24.
- Nwangburuka, C.C., O.B. Kehinde, D.K. Ojo, O.A. Denton and A.R. Popoola, 2011. Morphological classification of genetic diversity in cultivated okra, Abelmoschus esculentus (L) Moench using principal component analysis (PCA) and single linkage cluster analysis (SLCA). Afr. J. Biotechnol., 10: 11165-11172.
- Komolafe, R.J., O.J. Ariyo and C.O. Alake, 2021. Diversity in phenotypic traits and mineral elements of okra (Abelmoschus esculentus L. Moench) genotypes. Int. J. Agron., 2021.
- Gomurgen, A.N., 2005. Cytological effect of the poTASRsium metabisulphite and poTASRsium nitrate food preservative on root tips of Allium cepa L. Cytologia, 70: 119-128.
- Wang, Y., Y. Wei, X. Wang, Z. Wang and H. Wang, 2023. A clustering-based extended genetic algorithm for the multidepot vehicle routing problem with time windows and three-dimensional loading constraints. Appl. Soft Comput., 133.
- Saucedo, M.O., S.H.S. Rodríguez, C.F.A. Flores, R.B. Valenzuela and M.A.L. Luna, 2019. Effects of ultraviolet radiation (UV) in domestic animals. Review. Rev. Mex. Cienc. Pecu., 10: 416-432.
- Sripong, K., P. Jitareerat and A. Uthairatanakij, 2019. UV irradiation induces resistance against fruit rot disease and improves the quality of harvested mangosteen. Postharvest Biol. Technol., 149: 187-194.
- Khan, T.U., R.A. Laskar and B. Debnath, 2018. Studies on the effects of ultraviolet irradiation on pea (Pisum sativum L.). Int. J. Genom. Data Min., 2.
- Sengkhamparn, N., R. Verhoef, H.A. Schols, T. Sajjaanantakul and A.G.J. Voragen, 2009. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Res., 344: 1824-1832.
- Bharathi, T., S. Gnanamurthy, D. Dhanavel, S. Murugan and M. Ariraman, 2013. Induced physical mutagenesis on seed germination, lethal dosage and morphological mutants of ashwagandha (Withania somnifera (L.) Dunal). Int. J. Adv. Res., 1: 136-141.
- Çavuşoğlu, K., T.K. Macar, O. Macar, D. Çavuşoğlu and E. Yalçın, 2022. Comparative investigation of toxicity induced by UV-A and UV-C radiation using Allium test. Environ. Sci. Pollut. Res., 29.
- Zuk-Golaszewska, K., M.K. Upadhyaya and J. Golaszewski, 2003. The effect of UV-B radiation on plant growth and development. Plant Soil Environ., 49: 135-140.
- Al-Jarrah, O.B. and M.B. Al-Qazwini, 2009. Measuring and calculating of ultraviolet ray index reaching a section of Baghdad City. J. Univ. Anbar Pure Sci., 3: 161-165.
- Jarerat, A., C. Techavuthiporn, C. Chanchomsuek and H. Nimitkeatkai, 2022. Enhancement of antioxidant activity and bioactive compounds in eggplants using postharvest LEDs irradiation. Horticulturae, 8.
- Al-Shammari, A.M., 2015. Environmental pollutions associated to conflicts in Iraq and related health problems. Rev. Environ. Health, 31. https://doi.org/10.1515/reveh-2015-0024
- Zhang, W., H. Jiang, J. Cao and W. Jiang, 2021. UV-C treatment controls brown rot in postharvest nectarine by regulating ROS metabolism and anthocyanin synthesis. Postharvest Biol. Technol., 180.
- Banaś, A.K., P. Zgłobicki, E. Kowalska, A. Bażant, D. Dziga and W. Strzałka, 2020. All you need is light. Photorepair of UV-induced pyrimidine dimers. Genes, 11.
- Ahandani, E.A., M. Fazilati, A. Boghozian and M.A. Ahandani, 2020. Effect of ultraviolet (UV) radiation bonds on growth and chlorophyll content of Dracocephalum moldavica L herb. J. Biomol. Res. Ther., 8.
- Derebe, A.D., A.G. Roro, B.T. Asfaw, W.W. Ayele and A.K. Hvoslef-Eide, 2019. Effects of solar UV-B radiation exclusion on physiology, growth and yields of taro (Colocasia esculenta (L.)) at different altitudes in tropical environments of Southern Ethiopia. Sci. Hortic., 256.
- Darras, A.I., V. Demopoulos, I. Bali, O. Katsiloulis and E. Kratimenou, 2012. Brief Exposures of Ultraviolet-C (UV-C) Irradiation Improves Flowering of Ornamental Plants. In: XI International Symposium on Flower Bulbs and Herbaceous Perennials, Baktir, I., W.B. Miller and R. Kamenetsky (Eds.), ISHS, Leuven, Belgium, ISBN: 978-90-66056-06-0, pp: 95-101.
- Fina, J.P., F. Masotti, S.P. Rius, F. Crevacuore and P. Casati, 2017. HAC1 and HAF1 histone acetyltransferases have different roles in UV-B responses in arabidopsis. Front. Plant Sci. 8.
- Dukowic-Schulze, S., A. Harvey, N. Garcia, C. Chen and G. Gardner, 2022. UV-B irradiation results in inhibition of hypocotyl elongation, cell cycle arrest, and decreased endoreduplication mediated by miR5642. Photochem. Photobiol., 98: 1084-1099.
- Yamaga, I., A. Ikegaya, S. Nakamura, S. Yamazaki and T. Nakajima, 2021. Alleviation of fruit decay during the export and domestic storage of satsuma mandarin fruit through temperature treatment and ultraviolet-C irradiation via phytoalexin production. Hortic. J., 90: 286-295.
- Valenta, K., K. Dimac-Stohl, F. Baines, T. Smith and G. Piotrowski et al., 2020. Ultraviolet radiation changes plant color. BMC Plant Biol., 20.
- Grant, R.H., G.M. Heisler, W. Gao and M. Jenks, 2003. Ultraviolet leaf reflectance of common urban trees and the prediction of reflectance from leaf surface characteristics. Agric. For. Meteorol., 120: 127-139.
- Eskins, K., 1992. Light-quality effects on Arabidopsis development. Red, blue and far-red regulation of flowering and morphology. Physiol. Plant., 86: 439-444.
- Kumar, S., G. Katna and N. Sharma, 2019. Mutation breeding in chickpea. Adv. Plants Agric. Res., 9: 355-362.
- Alafiatayo, A.A., S. Ahmad and M. Maziah, 2014. Total antioxidant capacity, total phenolic compounds and the effects of solvent concentration on flavonoid content in Curcuma longa and Curcuma xanthorhhiza rhizomes. Med. Aromat. Plants, 3.
- Yoshikawa, H., C. Honda and S. Kondo, 2007. Effect of low-temperature stress on abscisic acid, jasmonates, and polyamines in apples. Plant Growth Regul., 52: 199-206.
How to Cite this paper?
APA-7 Style
Ehumadu,
R.C., Egbucha,
K.C., Nwoko,
M.C., Nkaa,
F.A., Ohaegbulam,
M.O., Nmezi,
S.N., Arochi,
V.O., Anoribe,
I.O., Ahaiwe,
M.C. (2024). Morphorgenic Effect of UV-Light on Two Abelmoschus esculentus (L.) Moench. (Okra) Accessions. Trends in Applied Sciences Research, 19(1), 213-224. https://doi.org/10.3923/tasr.2024.213.224
ACS Style
Ehumadu,
R.C.; Egbucha,
K.C.; Nwoko,
M.C.; Nkaa,
F.A.; Ohaegbulam,
M.O.; Nmezi,
S.N.; Arochi,
V.O.; Anoribe,
I.O.; Ahaiwe,
M.C. Morphorgenic Effect of UV-Light on Two Abelmoschus esculentus (L.) Moench. (Okra) Accessions. Trends Appl. Sci. Res 2024, 19, 213-224. https://doi.org/10.3923/tasr.2024.213.224
AMA Style
Ehumadu
RC, Egbucha
KC, Nwoko
MC, Nkaa
FA, Ohaegbulam
MO, Nmezi
SN, Arochi
VO, Anoribe
IO, Ahaiwe
MC. Morphorgenic Effect of UV-Light on Two Abelmoschus esculentus (L.) Moench. (Okra) Accessions. Trends in Applied Sciences Research. 2024; 19(1): 213-224. https://doi.org/10.3923/tasr.2024.213.224
Chicago/Turabian Style
Ehumadu, Rophina, Chinweokwu, Kenechukwu Chris Egbucha, Magnus Chinwendu Nwoko, Francis Agodichi Nkaa, Maryjane Onyinyechi Ohaegbulam, Stella Ngozi Nmezi, Vincent Okechukwu Arochi, Innocent Obumneme Anoribe, and Matthew Chiemerie Ahaiwe.
2024. "Morphorgenic Effect of UV-Light on Two Abelmoschus esculentus (L.) Moench. (Okra) Accessions" Trends in Applied Sciences Research 19, no. 1: 213-224. https://doi.org/10.3923/tasr.2024.213.224

This work is licensed under a Creative Commons Attribution 4.0 International License.


