Organic and Inorganic Nutrient Sources Effects on Soil Microbial Activity in Cultivated Soil
| Received 29 Apr, 2025 |
Accepted 02 Jun, 2025 |
Published 03 Jun, 2025 |
Background and Objective: Excessive use of inorganic fertilizers to boost crop yields often leads to resource wastage, environmental pollution, and long-term soil degradation, necessitating sustainable alternatives. This study evaluated the effects of organic (Super Gro, poultry manure, pig manure) and inorganic (NPK) nutrient sources on soil microbial activity and related parameters following incubation of cultivated soil. Materials and Methods: Soil respiration, microbial metabolic quotient (qCO2), carbon mineralization quotient (qM), microbial biomass carbon to organic carbon ratio (Cmic:Corg), and microbial carbon change rate quotient (qC) were measured at 3, 6, 9, and 12 days after incubation (DAI). Data collected from the experiment were subjected to Analysis of Variance (ANOVA), and treatment means were compared using the Tukey test at a 5% significance level. Results: Results showed that Super Gro significantly increased soil respiration and cumulative CO2-C production at 3 DAI compared to other treatments and the control, while NPK recorded the lowest CO2 evolution by the experiment’s end. Organic amendments elevated qCO2, indicating microbial stress, and enhanced carbon immobilization, whereas inorganic fertilizers suppressed microbial activity over time. No significant differences in qM or Cmic:Corg were observed among treatments by 12 DAI, though organic sources consistently supported higher microbial activity. Negative qC values at 6 and 9 DAI suggested carbon loss, which diminished by 12 DAI. Conclusion: This study underscores the potential of integrating organic nutrient sources to enhance soil health and promote sustainable agriculture.
| Copyright © 2025 Adejoro et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Farmers often use large amounts of inorganic fertilizers to achieve high crop yields. However, excessive application of these fertilizers wastes resources and contributes to environmental pollution, including water contamination. A single application of inorganic fertilizer can boost crop yield and increase soil nutrient availability. Yet, prolonged overuse may degrade soil quality and lead to nutrient imbalances1. Adopting scientific methods to optimize fertilizer use can enhance soil quality and support sustainable agriculture2. Partially replacing inorganic fertilizers with organic manures supplies crops with essential trace elements and improves soil properties, such as water retention, nutrient content, aeration, temperature regulation, and microbial activity3. These benefits emphasize the critical role of organic manures in promoting sustainable agricultural practices.
However, microbial populations are highly sensitive to soil conditions and respond quickly to changes in land use, cropping systems, and fertilizer applications, as evidenced by Zhou and Ding4, Bohme et al.5, Wang et al.6, and Yusuf et al.7. To evaluate soil fertility and quality, researchers employ microbial indicators such as soil microbial biomass carbon (SMBC), soil microbial biomass nitrogen (SMBN), microbial quotient (qMB), enzyme activity, and soil respiration8,9. Different studies have shown that fertilizer application practices significantly influence these microbial parameters10-12. Notably, organic fertilizers excel at enhancing SMBC, SMBN, and soil enzyme activity13. As a result, shifts in microbial communities can serve as predictors of how organic and conventional management practices impact ecosystems14-16.
Research further shows that organic amendments improve soil nutrient status, microbial activity, and productivity, while exclusive reliance on inorganic fertilizers may diminish microbial activity and soil productivity17. Organic farming also fosters soil structure formation18, boosts biodiversity19-21, and mitigates environmental stress22. Despite these insights, this study aims to specifically assess the effects of organic and inorganic nutrient sources on soil microbial activity following cultivation.
MATERIALS AND METHODS
Study area: The study duration was three months and was carried out at the Agricultural Development Programme centre (ADP) in Ibadan and the Federal University of Technology, Akure, Nigeria. The study started in December, 2020 and ended in February, 2021. This study was conducted in the Laboratory of Crop, Soil, and Pest Management at the Federal University of Technology, Akure. The soil used was collected from the field after the cultivation of black sesame at the Agricultural Development Programme (ADP) in Ibadan, Nigeria. It is located at 7°30'N and 3°54'E, 168 m with an annual temperature of 21 to 32°C, and a mean relative humidity from 61 to 83%23.
The initial chemical properties of the soil showed that the soil was acidic with a pH (H2O) value of 4.52. Furthermore, the organic matter contents of the soil (1.32 g/kg), organic carbon (0.77), nitrogen (0.08), available phosphorus (4.98 ppm), exchangeable Potassium (0.16), and Calcium (4.00 cmoL/kg), while Magnesium contents (2.00 cmoL/kg).
Experimental treatments and design: The treatments consisted of soil amended with chicken manure, pig manure, NPK (20:10:10), Super Gro (Neolife liquid organic fertilizer), and control (no soil amendment). In the laboratory soil sample was passed through a 2 mm sieve and adjusted to 60% water holding capacity (WHC). They were then stabilized at room temperature. The 500 g of the stabilized soil samples was transferred into glass jars. The jars were then made airtight and incubated at room temperature for 12 days. The moisture condition of the soils was maintained at 60% of maximum water holding capacity by the addition of sterile distilled water at periodic intervals throughout the incubation period. To achieve this, the initial weights of the jars were determined and recorded at the beginning of incubation. These weights were confirmed from time to time, and any deviation, which indicated moisture loss, was corrected by adding water to arrive at the original weight. The experiment was set up as a Completely Randomized Design (CRD) and each treatment was replicated three times.
Soil respiration measurements: Soil basal respiration was determined using the alkali sorption and titration method as described by Anderson and Domsch24. Three days before sampling, a 10 mL solution of 0.5 M NaOH was dispensed into a 50 mL beaker and placed inside the glass jars containing
the treated soil to trap CO2 evolved from the soil. On the third day, 5 mL of 1.0 M BaCl2 was added to the NaOH solutions from the jars to precipitate carbonate (as BaCO3), enabling the measurement of CO2 evolution (expressed as g CO2-C/g soil) from the treated soil. The evolved CO2-C was quantified by titration. Using phenolphthalein as an indicator, the NaOH solution was titrated against 0.5 M HCl. Two soil-free blanks were included to determine the amount of CO2 trapped in the absence of soil samples.
Determination of Eco-Physiological indices (qCO2, qM, qD/qC, and Cmic:Corg): At the end of the incubation period, eco-physiological indices, including qCO2 (Community respiration per biomass unit, or metabolic quotient), qM (Mineralization quotient), and Cmic:Corg, were calculated. The qCO2 was determined as the ratio of cumulative CO2-C (g CO2-C/g soil) to soil microbial biomass carbon, while qM was calculated as the ratio of CO2-C (g CO2-C/g soil) to soil organic carbon (mg/g soil). The Cmic:Corg ratio was derived from soil microbial biomass carbon and total organic carbon.
The microbial biomass change rate quotient (qC), which expresses the daily enrichment or loss of soil microbial carbon, was calculated based on the death rate quotient (qD) as reported by Anderson and Domsch24 and Adejoro et al.25. The C-loss quotient (unit C-loss/unit Cmic residual/h) was calculated based on total microbial carbon loss after the incubation period, using the following equation:
The qC was preferred over the qD of Anderson and Domsch24 because the treatments resulted in both carbon loss and enrichment.
Data analysis: Data collected from the experiment were subjected to Analysis of Variance (ANOVA), and treatment means were compared using the Tukey test at a 5% significance level.
RESULTS
The effects of various treatments on soil respiration from 3 to 12 days after incubation (DAI) are depicted in Table 1. Significant changes in soil respiration were observed only on the 3rd day after incubation (3 DAI). At 3 DAI, soil treated with Super Gro exhibited a significantly greater increase in soil respiration (43.50) compared to other treatments. From the 6th DAI until the end of the experiment, none of the treatments significantly (p<0.05) altered soil respiration. By the end of the incubation period, all treatments slightly reduced soil CO2-C production, though these reductions were not statistically significant. Among the treatments, NPK recorded the lowest (61.80) CO2 evolution at this stage.
Significant variations in cumulative soil respiration among treatments were observed on the 3rd day after incubation (Table 2). The presence of treatments significantly increased cumulative soil respiration compared to the control on 3 DAI. Throughout the incubation period, soil treated with Super Gro consistently produced the highest cumulative CO2-C at each sampling time, while the control treatment exhibited the lowest cumulative soil respiration. From 6 DAI until the termination of the experiment, none of the treatments significantly altered cumulative soil respiration.
| Table 1: | Treatment effects of organic and inorganic sources on basal soil respiration | |||
| Days after incubation | ||||
| Treatment | 3 | 6 | 9 | 12 |
| Chicken manure | 30.60b | 61.20a | 67.50a | 65.70a |
| Pig manure | 26.70b | 63.90a | 69.90a | 64.80a |
| N:P:K (20:10:10) | 25.80b | 65.10a | 69.00a | 61.80a |
| Super Gro | 43.50a | 64.50a | 68.10a | 66.00a |
| Control | 25.20b | 64.80a | 69.30a | 63.30a |
| Means in the same column with different superscript are significantly different using Tukey at (p<0.05) | ||||

|
|
| Table 2: | Treatment effects on the cumulative soil respiration | |||
| Days after incubation | ||||
| Treatment | 3 | 6 | 9 | 12 |
| Chicken manure | 30.60b | 91.80a | 159.30a | 225.00a |
| Pig manure | 26.70b | 90.60a | 160.50a | 225.30a |
| N:P:K (20:10:10) | 25.80b | 90.90a | 159.90a | 221.70a |
| Super Gro | 43.50a | 108.00a | 176.10a | 242.10a |
| Control | 25.20b | 90.00a | 159.30a | 222.60a |
| Means in the same column with different superscript are significantly different using Tukey at (p<0.05) | ||||
All treatments increased the microbial metabolic quotient (qCO2) compared to the control at 3 DAI (Fig. 1). Soil treated with Super Gro showed the highest increase in qCO2, followed by pig manure-treated soil, with both differing significantly (p<0.05) from the control. Super Gro maintained an elevated qCO2 until the end of incubation, followed by NPK. Significant differences were observed between 3 DAI and 12 DAI. The trend from 6 DAI to the end of incubation was as follows: Super Gro>NPK>Poultry manure>Pig manure>Control.
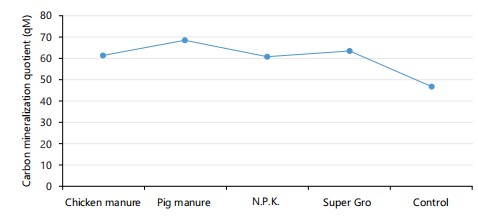
The rate of change in soil carbon mineralization (qM) among treatments was assessed at 3 DAI (Fig. 2). No significant differences were observed between the treatments and the control at this time. However, differences among treatments were evident, though their effects on the carbon mineralization quotient (qM) at the end of incubation were minimal. All treated soils exhibited an increase in the carbon mineralization quotient.

|

|
The effect of treatments on the ratio of biomass C to organic C is presented in Fig. 3. This parameter increased in the following order: Pig manure>Chicken manure>Super Gro>NPK>Control. The observed differences were not statistically significant (p<0.05). The trend remained unchanged from 6 DAI until the end of the experiment.
As shown in Fig. 4, the microbial carbon change rate quotient (qC) was evaluated on the 6th, 9th, and 12th days after incubation. At 6 DAI, negative qC values were recorded in both treated and untreated soils. Carbon loss was significantly (p<0.05) lower for control, NPK, Super Gro, chicken manure, and pig manure-treated soils compared to the other conditions. At 9 DAI, negative qC values persisted in treated soils, except for those treated with chicken manure and NPK, as well as the untreated soil. At 6 and 9 DAI, a death quotient (qD) scenario was observed across all treatments, including the control, with the percentage of C loss relative to untreated soil following the order: The NPK>Super Gro>Chicken manure>Pig manure. Furthermore, at 12 DAI, the trend shifted, with carbon loss becoming negligible for control, Super Gro, and NPK treatments. No carbon loss was recorded in any treatment by the end of this period.
DISCUSSION
The results from the research showed that organic and inorganic nutrient sources has effects on soil microbial activity and soil health in a cultivated soil. Soil respiration, an indicator of microbial activity, serves as a key measure of soil health26. In this study, the observed increase in soil respiration in rhizosphere soil amended with super Gro and chicken manure suggests that the nutrients supplied by these fertilizers stimulated the activity of native soil microorganisms. This finding aligns with previous research by Goyal et al.27 and Fontaine et al.28, which demonstrated that the addition of organic manures from plant and animal sources enhances soil respiration. Because of the breakdown of these integrated wastes, organic matter has been added to the soil, which is responsible for the notable rise in soil microbial biomass. Based on their research on manure boosting larger microbial biomass under tobacco growing, Ye et al.29 confirmed this by stating that residue amendments can encourage the massive reproduction of soil microorganisms and supply appropriate organic nutrition. One possible explanation for inorganic fertilizer inhibiting microbial activity is a decline in microbial respiration brought on by a dwindling microbial population. Conversely, organic materials such as animal manure, green manure, and crop residues improved soil nutrient status, microbial activity, and biodiversity. These observations are consistent with findings from multiple studies reporting that chemical fertilizers diminish soil productivity and microbial activity17,19.
Soils amended with organic and inorganic sources exhibited higher microbial metabolic quotients (qCO2) compared to the control. According to Anderson and Domsch24 and Adejoro et al.25, a rise in qCO2 may indicate that more carbon is lost in the form of CO2, but it also indicates high microbial activity and can be interpreted as a positive property. A high qCO2 is a clear indication of high maintenance carbon demand, and microbial biomass must decline if the carbon lost through respiration cannot be replenished within the soil system. The increase in qCO2 indicates that the indigenous microbial population expended more energy in the decomposition of the organic and inorganic sources.
Regarding the percentage of soil organic carbon mineralized, differences among treatments were minimal. Nonetheless, all treatments influenced carbon mineralization, as evidenced by the carbon mineralization quotient (qM). The ratio of microbial biomass carbon to organic carbon (Cmic:Corg) represents the capacity of soil microorganisms to mineralize and immobilize carbon. An increase in this ratio in the presence of both organic and inorganic amendments suggests that the soil microbiome immobilized more carbon, leading to greater biomass production. The carbon enrichment observed in soils amended with both organic and inorganic sources likely stems from the presence of easily mineralizable carbon in organic amendments, which soil microbes rapidly immobilize.
Microbial carbon loss, often expressed as the microbial death quotient (qD), was evident in this study and aligns with descriptions by Anderson and Domsch24 and Adejoro et al.25. This loss may result from the depletion of available carbon in the incubated soil samples over time.
CONCLUSION
The soil microbiological indicator was highly responsive to changes in soil processes triggered by the addition of organic and inorganic nutrient sources. Observations revealed a general increase in both microbial biomass and activity in the soil amended with organic and inorganic nutrient sources as compared to the control (non-amended). Moreover, results obtained under laboratory conditions suggest that the decomposition of organic and inorganic nutrient sources can be fairly predicted. Thus, the biological condition of the soil can be improved and the ecological balance of soil microbes supported by adding organic manure as a source of organic matter.
SIGNIFICANCE STATEMENT
The research aims to compare the effects of organic and inorganic nutrient sources on soil microbial activity and soil health. Organic nutrients improve the soil and help maintain microbial activity compared to inorganic nutrients. Farmers are employed to use organic nutrient sources to maintain their soil health and microbial activity for adequate, sustainable agriculture.
REFERENCES
- Chen, H., C.F. Cao, L.C. Kong, C.L. Zhang and W. Li et al., 2014. Study on wheat yield stability in Huaibei limeconcretion black soil area based on long-term fertilization experiment. Sci. Agric. Sin., 47: 2580-2590.
- Yadav, R.L., B.S. Daiwivedi, K. Prasad, O.K. Tomar, N.J. Shurpali and P.S. Pandey, 2000. Yield trends, and changes in soil organic-C and available NPK in a long-term rice-wheat system under integrated use of manures and fertilisers. Field Crops Res., 68: 219-246.
- Bolan, N.S., D.C. Adriano, R. Natesan and B.J. Koo, 2003. Effects of organic amendments on the reduction and phytoavailability of chromate in mineral soil. J. Environ. Qual., 32: 120-128.
- Zhou, L. and M. Ding, 2007. Soil microbial characteristics as bioindicators of soil health. Biodivers. Sci., 15: 162-171.
- Böhme, L., U. Langer and F. Böhme, 2005. Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agric. Ecosyst. Environ., 109: 141-152.
- Wang, X.L., Y. Jia, X.G. Li, R.J. Long, Q. Ma, F.M. Li and Y.J. Song, 2009. Effects of land use on soil total and light fraction organic, and microbial biomass C and N in a semi-arid ecosystem of Northwest China. Geoderma, 153: 285-290.
- Yusuf, A.A., R.C. Abaidoo, E.N.O. Iwuafor, O.O. Olufajo and N. Sanginga, 2009. Rotation effects of grain legumes and fallow on maize yield, microbial biomass and chemical properties of an alfisol in the Nigerian savanna. Agric. Ecosyst. Environ., 129: 325-331.
- Harris, J.A., 2003. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci., 54: 801-808.
- Schloter, M., O. Dilly and J.C. Munch, 2003. Indicators for evaluating soil quality. Agric. Ecosyst. Environ., 98: 255-262.
- Chu, H., X. Lin, T. Fujii, S. Morimoto, K. Yagi, J. Hu and J. Zhang, 2007. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol. Biochem., 39: 2971-2976.
- Jangid, K., M.A. Williams, A.J. Franzluebbers, J.S. Sanderlin and J.H. Reeves et al., 2008. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem., 40: 2843-2853.
- Xiaoxia, M., W. Lianlian, L. Qinghui, L. Hua, Z. Shulan, S. Benhua and Y. Xueyun, 2012. Effects of long-term fertilization on soil microbial biomass carbon and nitrogen and enzyme activities during maize growing season. Acta Ecol. Sin., 32: 5502-5511.
- Kautz, T., S. Wirth and F. Ellmer, 2004. Microbial activity in a sandy arable soil is governed by the fertilization regime. Soil Biol. Biochem., 40: 87-94.
- Bending, G.D., M.K. Turner, F. Rayns, M.C. Marx and M. Wood, 2004. Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol. Biochm., 36: 1785-1792.
- van Bruggen, A.H.C. and A.M. Semenov, 2000. In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol., 15: 13-24.
- Poudel, D.D., W.R. Horwath, W.T. Lanini, S.R. Temple and A.H.C. van Bruggen, 2002. Comparison of soil N availability and leaching potential, crop yields and weeds in organic, low-input and conventional farming systems in Northern California. Agric. Ecosyst. Environ., 90: 125-137.
- Kang, G.S., V. Beri, B.S. Sidhu and O.P. Rupela, 2005. A new index to assess soil quality and sustainability of wheat-based cropping systems. Biol. Fertil. Soils, 41: 389-398.
- Pulleman, M., A. Jongmans, J. Marinissen and J. Bouma, 2003. Effects of organic versus conventional arable farming on soil structure and organic matter dynamics in a marine loam in the Netherlands. Soil Use Manage., 19: 157-165.
- Doles, J.L., R.J. Zimmerman and J.C. Moore, 2001. Soil microarthropod community structure and dynamics in organic and conventionally managed apple orchards in Western Colorado, USA. Appl. Soil Ecol., 18: 83-96.
- Maeder, P., A. Fliessbach, D. Dubois, L. Gunst, P. Fried and U. Niggli, 2002. Soil fertility and biodiversity in organic farming. Science, 296: 1694-1697.
- Oehl, F., E. Sieverding, P. Mäder, D. Dubois, K. Ineichen, T. Boller and A. Wiemken, 2004. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia, 138: 574-583.
- Horrigan, L., R.S. Lawrence and P. Walker, 2002. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect., 110: 445-456.
- Oduntan, A.O., O. Olaleye, B.A. Akinwande and S.B. Fasoyiro, 2014. Effect of plant maturity on the antinutritent of Sesamum radiatum leaves. Global J. Sci. Res., 2: 7-11.
- Anderson, T.H. and K.H. Domsch, 1990. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem., 22: 251-255.
- Adejoro, S.A., A.C. Adegaye and D.S. Sonoiki, 2018. Soil microbial community response to compost addition to nicosulfuron contaminated soil. J. Agric. Stud., 6: 49-63.
- Nannipieri, P., J. Ascher, M.T. Ceccherini, L. Landi, G. Pietramellara and G. Renella, 2003. Microbial diversity and soil functions. Eur. J. Soil Sci., 54: 655-670.
- Goyal, S., K. Chander, M.C. Mundra and K.K. Kapoor, 1999. Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol. Fertil. Soils, 29: 196-200.
- Fontaine, S., A. Mariotti and L. Abbadie, 2003. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem., 35: 837-843.
- Ye, X., H. Liu, Z. Li, Y. Wang, Y. Wang, H. Wang and G. Liu, 2014. Effects of green manure continuous application on soil microbial biomass and enzyme activity. J. Plant Nutr., 37: 498-508.
How to Cite this paper?
APA-7 Style
Adejoro,
S.A., Olusola,
O.J., Alade,
O.D. (2025). Organic and Inorganic Nutrient Sources Effects on Soil Microbial Activity in Cultivated Soil. Trends in Applied Sciences Research, 20(1), 48-55. https://doi.org/10.3923/tasr.2025.48.55
ACS Style
Adejoro,
S.A.; Olusola,
O.J.; Alade,
O.D. Organic and Inorganic Nutrient Sources Effects on Soil Microbial Activity in Cultivated Soil. Trends Appl. Sci. Res 2025, 20, 48-55. https://doi.org/10.3923/tasr.2025.48.55
AMA Style
Adejoro
SA, Olusola
OJ, Alade
OD. Organic and Inorganic Nutrient Sources Effects on Soil Microbial Activity in Cultivated Soil. Trends in Applied Sciences Research. 2025; 20(1): 48-55. https://doi.org/10.3923/tasr.2025.48.55
Chicago/Turabian Style
Adejoro, Solomon, Alaba, Olusegun James Olusola, and Olakanmi David Alade.
2025. "Organic and Inorganic Nutrient Sources Effects on Soil Microbial Activity in Cultivated Soil" Trends in Applied Sciences Research 20, no. 1: 48-55. https://doi.org/10.3923/tasr.2025.48.55

This work is licensed under a Creative Commons Attribution 4.0 International License.


