Sorption of Selected Metals from Cement Factory Wastewater using Alkali Activated Carbons Derived from PET (Polyethylene terephthalate)
| Received 12 Feb, 2023 |
Accepted 20 Jun, 2023 |
Published 07 Aug, 2023 |
Background and Objective: Cocoa husk and Polyethylene terephthalate (PET) are solid wastes that need to be sustainably managed. This study evaluated the effect and efficiency of Cocoa Ash alkali-polyethylene terephthalate (CAPET) and KOH PET activated carbons (ACs) for removal of selected metals (Ca, Cr, Zn, Mn, Cu and Fe) from cement factory wastewater. Materials and Methods: Scanning Electron Microscope coupled with Energy Dispersive X-ray Spectrophotometer (SEM/EDX) and Fourier Transform Infrared Spectrometer (FTIR) were used to characterize the ACs. Using ICP-OES, the initial and final concentrations of metals present in wastewater were determined. Langmuir and Freundlich sorption isotherm models were applied to determine metals’ interface with alkali-PET adsorbents. Results: The carbon yield of alkali-PET (CAP and KOH PET) ACs were 81.4 and 91.1%, respectively. The EDX analysis of the ACs also resulted in carbon having the highest composition. The results of the adsorption using CAPAC and KPAC as adsorbents revealed that concentrations of some metals (Cr, Zn, Mn, Cu and Fe) were below detection level (0.00 mg L‾1) in both upstream and downstream wastewater samples, with the exception of Calcium (Ca). This implies 100% adsorption efficiency for metals (Cr, Zn, Mn, Cu and Fe) and 95% for Ca. The Freundlich adsorption isotherm fits the experimental data better than the Langmuir adsorption isotherm. Conclusion: The study indicated that rather than polluting the environment, PET and cocoa husk might be processed to act as low-cost, non-hazardous adsorbents for eliminating metal pollutants from wastewater.
| Copyright © 2023 Okoya et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Globally, Industrial activities have been associated, among many other anthropogenic processes, as one of the possible sources of hazardous metals in the environment1. In developing countries, a vast majority of wastewater is released, either before or after traditional treatment, into the environment. As a result of such poor wastewater treatment procedures, the organic load discharged into receiving waters is frequently quite high and chemically complex2. The Central Pollution Board lists the cement business as one of the most polluting industries, with key pollutants such as noise, dust and wastewater generation. Pollutants are released at every stage of the manufacturing process, including raw material extraction, crushing, manufacture and so on. The elements content of soil and the physicochemical parameters of receiving water bodies are altered by cement dust and wastewater3. It is also a major source of particulate matter emissions such as SOx, NOx and CO2. Heavy metals such as chromium, nickel, cobalt, lead and mercury are found in the dust and wastewater produced which are harmful to the environment and have an impact on human and animal health. Carcinogenesis, poor antioxidant capacity, acute respiratory symptoms and acute ventilatory effects have all been linked to cement wastewater in humans and animals.
Due to their toxicity, persistence, accumulation and non-biodegradability, heavy metal contamination has been a severe environmental concern. This contamination has risen in recent decades as a result of the rising population, which has boosted consumption rates and accelerated industrialization, resulting in increased production. Metals are released into the air, soil and water as a result of industrial processes4. It has long been a goal to reduce their concentrations in industrial and municipal effluents before they are released into the environment5. To avoid one of the major sources of water and soil pollution, removing poisonous and damaging metal ions from industrial effluents, water supplies and mine fluids is a serious task.
Ion-exchange resins, adsorption, electrolytic or liquid extraction, electro-dialysis, chemical precipitation, membrane filtration, biosorption and chelating resins have all been proposed as ways for removing heavy metal ions from wastewaters6. Adsorbents play a critical role in the efficiency of an adsorption process. Most modern adsorbents for the removal of heavy metal ions rely on interactions with functional groups on the adsorbents’ surfaces and hence the functional groups have a significant impact on the adsorbents’ effectiveness, capacity and reusability6.
Activated carbon is one of the most often utilized adsorbents in the treatment of industrial effluents because it can adsorb a large number and diversity of pollutants on its surface, concentrating and eliminating them from the effluent. Wood, mineral carbon, coconut shell, animal bones and polymers such as PET7 are the basic materials historically utilized in the industrial production of activated carbon. Due to the high volume of solid waste produced, the discharge of PET waste poses a severe issue for waste management solutions. Environmental restrictions have made it necessary to find suitable alternatives that may be reused or recycled. Hence, this study seeks to develop adsorbents from Cocoa husk and PET which are solid wastes that need to be sustainably managed to remove metals (particularly heavy metals) from cement factory wastewater. The caustic alkali is made from cocoa pod husk, an agricultural solid waste, is compared to the imported and expensive analytically graded KOH as an alternative chemical activation agent in the production of PET-activated carbons.
MATERIALS AND METHODS
Sample collection and preparation of pet activated carbons
Study area: The study was carried out between 2020 and 2021 at the SwWECh laboratory, Institute of Ecology and Environmental Studies, Obafemi Awolowo University, Ile-Ife, Nigeria.
Sample collection: The cocoa husks for this study came from the Obafemi Awolowo University Teaching and Research Farm, while the PET bottle waste came from waste bins in Ile-Ife, Osun. The cocoa husks were sun-dried, pulverized and sieved with less than 150-micron mesh size sieve to improve the surface area for cocoa ash production7, while the PET bottle wastes were washed, air-dried and cut into very small sizes8.
Preparation of cocoa husk ash: To obtain ash, pulverized cocoa husk of a known weight (20 kg) was placed in a weighted clean dried crucible and placed in a furnace (Carbolite 12/65 tube furnace, ESSEN Germany) at 600°C for 8 hrs. In the open air, the crucibles containing the ash were allowed to cool to ambient temperature. After cooling, the materials were placed in a desiccator7.
Preparation of caustic alkali from cocoa husk ash: Deionized water (1000 mL) was added to 190 g of cocoa husk ash. The resulting mixture was manually stirred and shaken vigorously. It was then filtered with a glass funnel and Whatman filter paper (No. 1) to obtain an alkali (filtrate) solution of described by Maliki et al.7.
Activation of PETs utilizing alkali filtrate and analytical graded KOH: The method is as outlined by Okoya et al.8 and Sabir et al.9, The alkali obtained (106.37 g) from the filtrate was added to a PET weight of 53.16 g at the ratio 2:1 in 1000 mL deionized water. Also, analytical graded KOH (106.3 g) was added to 53.16 g PET weight at the same ratio in 1000 mL deionized water. Each of the mixtures was thoroughly mixed manually and boiled gently over a magnetic stirrer with a hotplate (Stuart Scientific) operated at the temperature of 85°C for 6 hrs at SwWECh Laboratory and Research Unit, Institute of Ecology and Environmental Studies, Obafemi Awolowo University, Ile-Ife, Osun State. It was then dried in a laboratory drying oven (DHG-9030) at 110°C until total evaporation was achieved and stable weights were obtained. The dried samples were then washed with 0.01M HCl to remove the unreacted alkali until neutral pH was achieved. The samples were finally dried at room temperature.
Carbonization of the activated PETs: The activated PETs (CAP and KOH PET) were weighed and carbonized in a muffle furnace (Carbolite 12/65 tube furnace, ESSEN Germany). The furnace’s temperature was set to 500°C and maintained for one hour. The crucibles containing the charred materials were removed from the furnace after they had cooled. The carbon yield was calculated by weighing the cooled charred materials and the operation was repeated three times. The percentage carbon yield was calculated utilizing Eq. 1:
| (1) |
The charred materials referred to as PET activated carbons were further ground into powder utilizing agate mortar and pestle, sieved (<150 μs) and characterized7.
Characterization of the activated carbons: The dry combustion technique was used to assess the surface morphological features and elemental composition of the ACs using a scanning electron microscope and an energy-dispersive X-ray analyzer (high-resolution SEM/EDX, JOEL-JSM 7600F). The Fourier transform infrared (SHIMADZU-FTIR-8400S) approach was used to determine the structural chemical functional groups in the ACs. The activated carbons’ pH and ash content were also assessed.
Preparation of samples for SEM/EDX analysis: The methods were detailed as in Okoya et al.10, using a double-sticky carbon tape, the alkali PETs (CAP and KOH) ACs samples were adhered to an aluminum frame stub. Carbon was used to insulate the samples, which were then electrically grounded. Similarly, silver paint was used to ground the samples electrically. The samples were then dried thoroughly in a drying oven at 60°C for 3 hrs. The SEM machine was turned on once the samples were placed into the frame. The samples were then placed in a relatively high-pressure chamber with a short working distance and a differentially pumped electron optical column to keep the vacuum at the electron cannon low enough. After then, the footage was obtained. After that, the energy dispersive X-ray acceleration voltage was set to 20 kV with a working distance of 14 mm, the detector was rotated to 45 mm, the samples were focused and the X-ray spectrum was collected and recorded.
Preparation of samples for FTIR analysis: The FTIR KBr technique was used to examine the alkali PET ACs samples. Using a micro spatula, a very small amount of the sample (approximately 1/8") was taken and 0.25 to 0.50 teaspoons of KBr were completely mixed in a mortar while being ground with a pestle. The KBr-sample mixture was pelletized, fixed into a sample container connected to a recording device and analyzed.
Determination of pH of the adsorbents: The pH was determined using a HI9812-5 portable pH/EC/TDS temperature meter. In a 200 mL beaker, the activated PET carbons (2.0 g) were weighed, 100 mL deionized water was added and the mixture was gently boiled for 10 min. The solution was cooled at room temperature. Finally, it was gently stirred for a few minutes and the pH was determined immediately10,11.
Determination of ash content of the adsorbents: The ash content was determined by weighing 20 g of the alkali PET ACs. The ACs were burned for 4 hrs in a muffle furnace at 500°C. It was then weighed after cooling in a desiccator. Equation (2) for determining ash content is presented12:
| (2) |
Sampling and sample preparation of industrial effluent: The industrial effluent sample was collected from the discharge point, upstream and downstream in triplicate from the three sampling points. A homogeneous mixture of the discharge point wastewater sample was made by mixing an equal proportion of the wastewater sample, also the upstream and downstream wastewater samples were homogenized separately following the same procedure. To prevent heavy metal precipitation, the industrial wastewater samples were treated with 2 mL nitric acid (HNO3)13,14. Using inductively coupled plasma optical emission spectroscopy, the metal content of wastewater samples was evaluated both before and after adsorption (ICP-OES).
Digestion of heavy metals in industrial effluent: To the acidified industrial effluent sample 50 mL, 5 mL of 65% HNO3 was added and the mixture was boiled gently utilizing a magnetic stirrer with a hotplate (Stuart Scientific) operated at the temperature of 85°C until complete digestion (when a clear solution is obtained)15. Using an inductively coupled Plasma Optical Emission Spectrophotometer (ICP-OES Agilent 720, Santa Clara, California, United States), the metal content of the digested industrial effluent was assessed.
Batch adsorption experiment of simulated wastewater: The method outlined in Okoya et al.14 and Bhatti et al.16 was used to investigate batch adsorption tests. Metal-contaminated water (Ca, Cr, Zn, Mn, Cu and Fe) was replicated in the lab by making a separate stock solution for each metal and diluting it into various quantities (0.01, 0.20, 5, 10, 15 and 20 mg L–1). Batch adsorption tests were conducted using 0.25, 0.5, 0.75 and 1.0 g of alkali PET (CAP and KOH) ACs as the adsorbent. Each simulated solution of metals (10 mL) polluted water was placed in a separate conical flask and subjected to constant shaking with an orbital shaker (Celtech KJ-201BD) set to 120 osc/min. For effective adsorption, the initial metal concentrations, pH, contact time and sorbent dosage were tuned. Following the batch adsorptions, Whatman filter paper was used to filter the samples (No. 1). The metal concentrations in the filtrates containing residual concentrations of the metals (Ca, Cr, Zn, Mn, Cu and Fe) were evaluated using the Inductively Coupled Plasma Optical Emission Spectroscopic method (ICP-OES) Agilent 720.
Statistical analysis: One-way analysis of variance was used to analyze the data (ANOVA). The distribution of one factor among numerous independent groups or means was investigated using one-way ANOVA at p = 0.05.
Adsorption efficiency: The amount of metals (Ca, Cr, Zn, Mn, Cu and Fe) adsorbed by a unit mass of the adsorbents (CAP and KOH PET ACs) and the percentages of metals (adsorption efficiency percent) adsorbed were determined for each adsorption procedure and were computed using the Eq. (3)8,17:
| (3) |
| C0 | = | Initial concentration before adsorption | |
| C1 | = | Final concentration of a metal solution (mg L–1) in the filtrate after adsorption |
RESULTS AND DISCUSSION
Collection of samples and preparation of cocoa husk alkaline solution: The PET was found littering the environment as wastes and some were found blocking waterways and these were collected at no extra cost apart from transporting them to the laboratory. Also, no extra cost was involved in the collection of cocoa husk apart from transporting same to the laboratory. This indicates that these solid wastes are a nuisance to the environment and that they must be processed into valuable products such as adsorbents for water and wastewater treatment. The cocoa husk powder 20 kg produced 2.03 kg of ash and the produced ash gave alkali filtrate when mixed with water. This is in agreement with the earlier report of Maliki et al.7 in which cocoa husk was produced ash and the resulting solution was alkaline in nature.
Characterization of alkali-PET ACs: Results of the investigation of the carbon yield, pH and ash contents of alkali-PET ACs (CAP and KOH PET) were shown in Table 1. A high percentage carbon yield was obtained for both CAP and KOH PET ACs (81.4±0.141 and 91.1±0.141, respectively). The PET appears to be a promising material to use as a precursor in the synthesis of ACs because of its high carbon and hydrogen content and low nitrogen, sulfur and ash content18. With the proven fact that high carbon yield indicates effective adsorbent10,19, high carbon yield combined with low ash content encourages superior pore structure characteristics. Similarly, the pH (6.8±0.00 and 6.7±0.141) which is very close to neutral coupled with the high carbon yield and low ash content is a reflection that the preparation method has a remarkable and significant influence on the mass and quality of the final product.
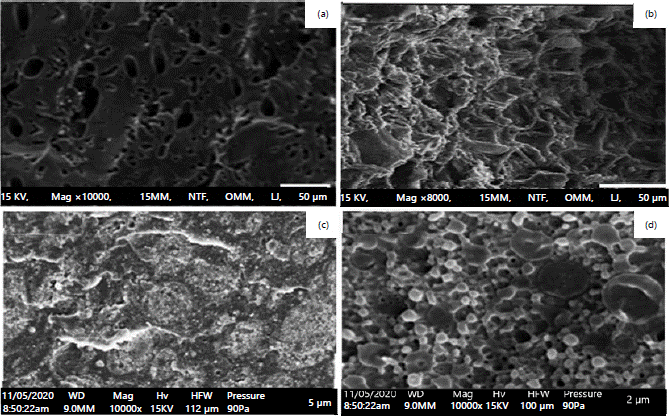
Surface morphology of the alkali (CAP and KOH PET) ACs before and after adsorption experiment: Surface properties and physical morphologies of the ACs by the scanning electron microscopy technique (SEM) were shown in Fig 1. The SEM is a vital instrument for determining the adsorbent surface shape and fundamental physical properties20. Figure 1a-b depicts the surface morphology prior to the adsorption processes shown in Fig. 1a-b, whereas Fig. 1c-d, depict the alkali ACs (adsorbent) surface morphology after the adsorption processes, as shown in Fig. 1c-d. It was observed that Fig. 1a-b, showed pore structure. However, following adsorption with the effluent, upstream and downstream samples, the pores displayed by the adsorbents have reduced drastically as shown in Fig. 1c-d, which may be a result of heavy metals blockage indicating that adsorption had occurred19,21.
Elemental composition of the activated carbons before and after adsorption: The elemental composition of the alkali-PET ACs was determined both before and after adsorption using a scanning electron microscope coupled with an energy-dispersive X-ray analyzer (SEM/EDX) (Table 2). For CAP and KOH PET ACs, the result demonstrated that carbon has the maximum percentage (55 and 60%, respectively) before adsorption while 70.09, 65.27 and 40.27% and 60.25, 70.00 and 43.20% for affluent, upstream and downstream wastewater after adsorption/treatment with CAP and KOH PET ACs, respectively of all the elements present and this makes the alkali-PET ACs (CAP and KOH PET) suitable for adsorption19,22. The use of oxygen to oxidize the organic components present could be linked to the strategic decrease in the percentage of oxygen (O) in the adsorbent after the adsorption process. Furthermore, the presence of hydrogen (H), iron (Fe), calcium (Ca) and silicon (Si) in the adsorbents upstream and downstream following the adsorption process could indicate that alkali ACs are similarly effective in removing these elements from wastewater samples (Table 2).
|
| Table 1: | Char yield and physicochemical parameters of CAP and KOH-PET activated carbon | |||
| Materials | Parameter |
SD (%) |
| CAP | Carbon yield |
81.4±0.141 |
pH |
06.8±0.000 |
|
Ash content |
02.6±0.000 |
|
| KOH-PET | Carbon yield |
91.1±0.141 |
pH |
06.7±0.141 |
|
Ash content |
01.5±0.000 |
|
| *SD: Standard deviation ±, CAP: Caustic alkali PET activated carbon and KOH-PET: KOH PET activated carbon | ||
| Table 2: | Elemental composition of the adsorbents (CPAC and KPAC) | |||
Before adsorption (%) |
After adsorption (%) |
|||||||
| Elements | CPAC |
KPAC |
EW (CPAC) |
EW (KPAC) |
USW (CPAC) |
USW (KPAC) |
DSW (CPAC) |
DSW (KPAC) |
| C | 55 |
60 |
70.09 |
60.25 |
65.27 |
70 |
40.27 |
43.2 |
| O | 20 |
30 |
15.66 |
15.5 |
10.48 |
9.27 |
10.48 |
10.36 |
| H | - |
- |
10.85 |
15.65 |
20.35 |
15.55 |
8.35 |
11.07 |
| S | 25 |
10 |
3.4 |
8.6 |
3.9 |
3.7 |
4.2 |
2.97 |
| Fe | - |
- |
- |
- |
- |
1.48 |
- |
- |
| Ca | - |
- |
- |
- |
- |
- |
12.2 |
7.4 |
| Si | - |
- |
- |
- |
- |
- |
25.3 |
25 |
| CPAC: Cocoa-PET activated carbon, KPAC: KOH-PET activated carbon, EW: Effluent wastewater, USW: Upstream wastewater and DSW: Downstream wastewater | ||||||||
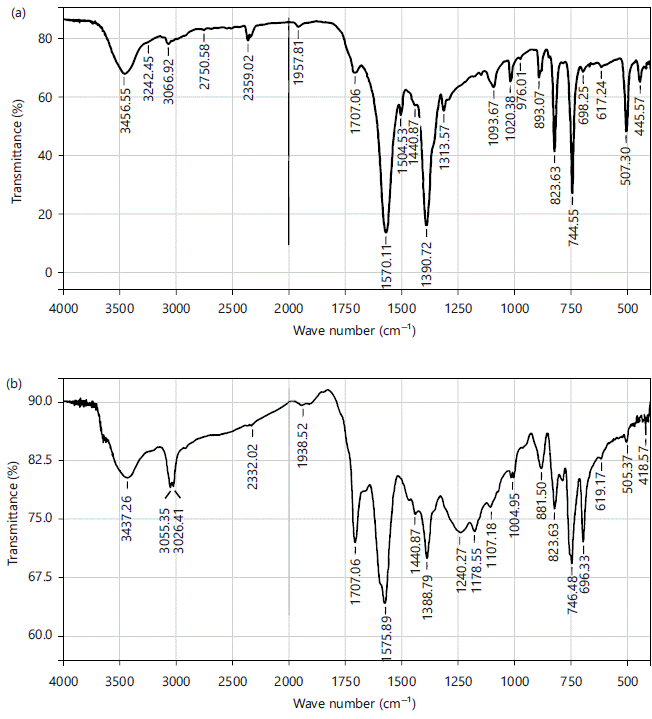
Functional groups of the alkali (CAP and KOH PET) ACs: Using Fourier transform-infrared spectroscopy (SHIMADZU-FTIR-8400S), the functional groups of the alkali-PET ACs (CAP and KOH ACs) were determined. The functional groups present in the CAP and KOH PET ACs were shown in Fig. 2(a-b). Each of the FTIR bands reflects a functional group that facilitates and supports adsorption. The Hydroxyl (3456.55 and 3066.92 cm–1), Aldehyde (2750.58 and 2359.02 cm–1), Aromatic (1957.81 and 1570.11 cm–1), Carbonyl (1504.532 and 1440.87 cm–1), Carbonate ester (1390.72 and 1313.57 cm–1), Alkene (1093.67 and 976.01 cm–1), Ester (893.07 and 698.25 cm–1) and Alkynes group (617.24 and 445.57 cm–1) functional groups are found in CAPAC while in the KOH PET AC are Hydroxyl (3437.26 and 3026.41 cm–1), Methoxy (2332.02 cm–1), Aromatic (1938.52 cm–1), Carbonyl (1707 and 1575.89 cm–1), Ether (1440.87 cm–1 and 1388.79 cm–1), Alkanes(1240.27 and 1107.18 cm–1), Alkene (1004.95 and 881.50 cm–1), Ester (823.63 and 619.17 cm–1) and Ether (505.37 and 418.57 cm–1).
|
Heavy metal content of cement industry wastewater samples before adsorption: The wastewater samples’ metal content was examined before the adsorption experiment to assess the range of metal pollutants. For the batch adsorption simulation investigation, all metals (Cr, Zn, Mn, Cu and Fe) that were above the NESREA standard limits (Table 3) were chosen.
Batch adsorption studies on simulated metal solutions utilizing alkali-Pet ACs as adsorbent: The effectiveness of alkali-PET ACs (CPAC and KPAC) as adsorbents for metals (Ca, Cr, Zn, Mn, Cu and Fe) adsorption was examined. Using the adsorbents, the effects of starting metal concentrations, pH, adsorbent dosage and contact time on adsorption were investigated. The following are the results of the effects of each of the parameters.
|
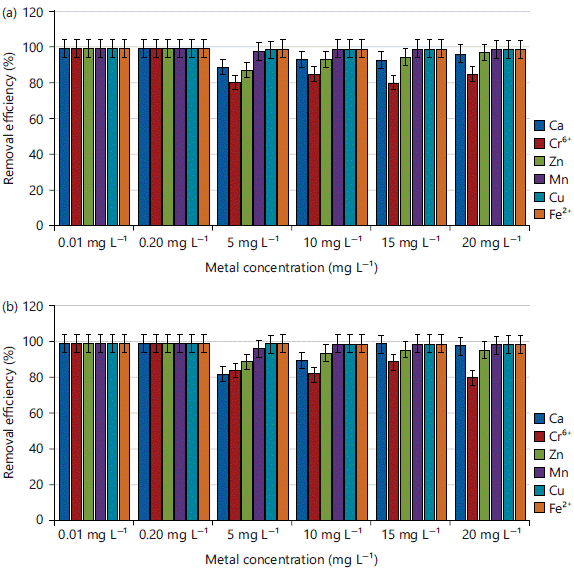
Effect of initial metal concentrations on the adsorption of Ca, Cr, Zn, Mn, Cu and Fe in simulated experiment: The findings of using CPAC and KPAC to investigate the adsorption of Ca, Cr, Zn, Mn, Cu and Fe with varying initial metal concentrations (0.01, 0.2, 5, 10, 15 and 20 mg L–1) were presented in Fig. 3a-b. while other conditions were kept constant. The percentage removal efficiencies obtained decreased with an increase in initial metal concentrations for CPAC and KPAC, respectively. The highest adsorption efficiency was observed at concentrations of 0.01 and 0.20 mg L–1 (Ca, Cr, Zn, Mn, Cu and Fe) while for both CPAC and KPAC, the lowest adsorption efficiency was found at 5 mg L–1. The same pattern was observed by Alwaan and Jaleel23. This trend can be explained in terms of the principle that once the surface of the adsorbent is nearly clogged with adsorbates, a second mechanism of intra-particle diffusion will be activated, inducing more adsorption but at a much slower and time-consuming rate because the reaction takes place inside the carbon matrix24.
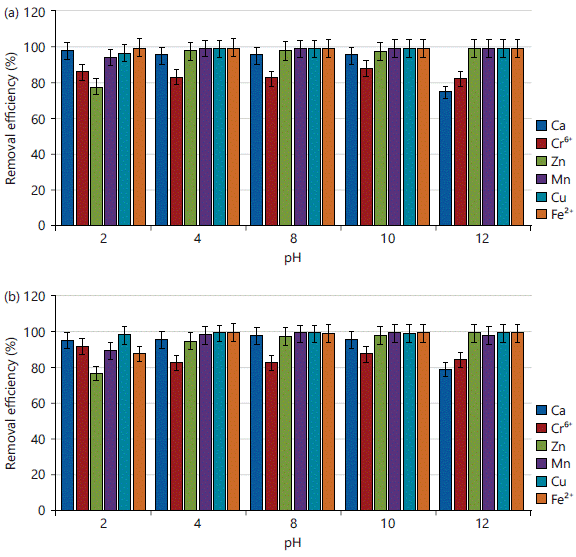
Effect of pH on the adsorption of Ca, Cr, Zn, Mn, Cu and Fe in simulated experiment: The pH of an aqueous solution is an essential operational parameter in the adsorption process because it has the ability to alter the adsorption modifier of ionized compounds10. Figure 4a-b showed the findings of the examination of the adsorption of Ca, Cr, Zn, Mn, Cu and Fe using CPAC and KPAC at different pH (2, 4, 8, 10 and 12), while all other variables were held constant. The results revealed that adsorption was favorable at both acidic (pH 2 and 4) and alkali (pH 8, 10 and 12) mediums. It can be emphasized that low pH (acidic) encourages the solubility of heavy metals. Higher (alkaline) and lower (acidic) pH values favour adsorption efficiency, this is consistent with the findings of Velásquez and Dussan25, Lukman et al.26 and Zhang et al.27 on the effects of pH on heavy metal adsorption. This could be because of the adsorbent’s hydroxyl bonds. According to Zhang et al.27, when the amount of the basic functional group of activated carbon increases, the hydrophilicity improves and the water vapor in the lower relative pressure range also increases. Therefore, it was revealed that a considerable reduction of metal (Ca, Zn, Cr, Mn, Cu and Fe) concentration is obtained in the pH range of 2 and 12, but with a slight reduction in efficiency for Zn at acidic pH (pH of 2) for both adsorbents.
|
Effect of contact time on Ca, Cr, Zn, Mn, Cu and Fe adsorption in a simulated experiment: Figure 5a-b showed the results of a study on the adsorption of metals (Ca, Cr, Zn, Mn, Cu and Fe) on CPAC and KPAC with different contact times (15, 30, 45 and 60 min), while other variables (pH, adsorbent dosage and concentration) were constant. The adsorption effectiveness rose as the contact period increased. The efficiency initially increased (Fig. 5a-b) and then progressively stayed constant as the contact time increased. In light of this, a contact period of 15 min is regarded as the equilibrium time with Ca (100%), Zn (99.70%), Cr (80.15%), Mn (99.90%), Cu (99.80%), Fe (99.95%) for CPAC and Ca (95.19%), Zn (95.57%), Cr (81.79%), Mn (99.34%), Cu (99.64%), Fe (100%) for KPAC. These findings could be explained by the presence of a significant number of unoccupied sites on the adsorbent’s surface at the start. These results could be explained by the presence of a significant number of unoccupied sites on the adsorbent’s surface at the start. The adsorption rate is rapid thereby increasing the amount of adsorbates adhering to the carbon surface majorly within 15 min of adsorption. This finding is consistent with the findings of Sugashini and Gopalakrishnan28, who investigated the performance of protonated cross-linked chitosan beads (PCCB) for chromium removal and Alwaan and Jaleel23, who investigated the production of high-quality activated carbon from PET bottle waste.
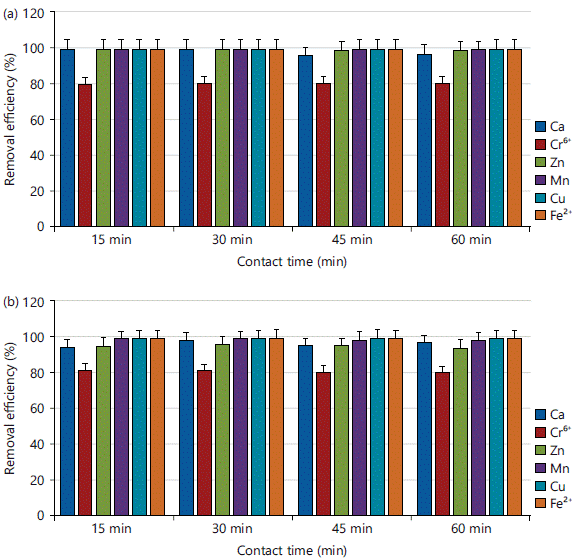
|
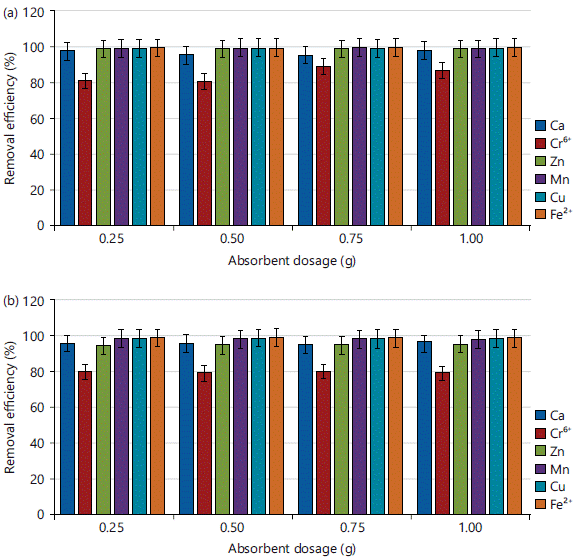
Effect of sorbent dosage on the adsorption of ca, cr, zn, mn, cu and fe in simulated experiment: Adsorbent dosage is a parameter used to determine an adsorbent’s ability to bind heavy metals at a given initial concentration. Figure 6a-b. showed the results of varying sorbent doses for the adsorption of heavy metals (Ca, Zn, Cr, Mn, Cu and Fe) using CPAC and KPAC as adsorbents, while all other parameters were maintained constant. The influence of sorbent dosages on Ca, Zn, Cr, Mn, Cu and Fe removal efficiency demonstrated that adsorption was efficient even at a minimum sorbent dosage of 0.25 g of CPAC and KPAC, achieving their equilibrium removal efficiency. However, from an economic standpoint, this means that for this study, 0.25 g of adsorbent was regarded as the optimal dosage for CAP and KOH PET ACs.
Adsorption study on industrial wastewater: Adsorption experiments on wastewater samples using the optimum value obtained from a simulation experiment using CPAC and KPAC as adsorbents revealed that metal concentrations (Cr, Zn, Mn, Cu and Fe) were below the detection level (0.00 mg L–1), with the exception of Calcium (Ca), which was higher (0.9 mg L–1) than the acceptable limit (0.5 mg L–1) in the effluent, upstream and downstream (Table 3). This implies 100% adsorption efficiency for metals (Cr, Zn, Mn, Cu and Fe) and 95% for Ca. The result indicated that CPAC and KPAC demonstrated excellent results for the adsorption of metals. The results of the adsorption experiment of Zn, Cr, Mn, Cu and Fe in effluents, upstream and downstream wastewater samples were below detection limits (0.00 mg L–1), while Ca reduced drastically to 0.968, 0.881 and 0.675 mg L–1 with CPAC and 0.932, 0.9096 and 0.771 mg L–1 with KPAC, respectively. Other metals present in the effluent, such as K, V, Hg, Ti, Ta and Ag, showed a decline in concentrations following adsorption, despite the fact that they were initially below their allowed limits. This shows the feasibility of the adsorbents in the adsorption of the heavy metals of interest (Cr, Zn, Mn, Cu and Fe), as well as other elements present in the wastewater samples vying for adsorption sites with the adsorbents. According to de Castro et al.19, ACs made of Polyethylene terephthalate (PET) eliminated a higher percentage of heavy metals from both industrial effluent and domestic waters. This result also showed that activated carbon made from PET is a good adsorbent for removing metals from wastewater, just like agriculturally based activated carbon, as reported by many researchers, including Bernard et al.29, who worked on heavy metals removal from industrial wastewater using activated carbon made from coconut shell.
|
According to the findings, activated carbon made from coconut shells is an effective adsorbent for removing lead, iron, copper and zinc ions. Table 3 showed that following the adsorption, the concentrations of all metals in the upstream, effluent and downstream samples were reduced. This confirms the suitability for the adsorption of metals of the alkaline PET ACs (sorbents) used. It will be good to put on record that the relatively low Ca concentration in the effluent compared to the upstream and downstream (Table 3) could be due to the cumulative effect of Ca in the stream environment, Ca being a major metal pollutant in a cement industry environment. Except for Ca, all the selected metals under study fall within the NESREA standard limits after the adsorption experiment (Table 3). However, the percentage reduction of Ca was also remarkable.
In this investigation, the removal efficiencies of both CPAC and KPAC were shown to be competitive, resulting in an outstanding efficiency for the removal of heavy metals (Ca, Zn, Cr, Mn, Cu and Fe). This demonstrates that alkaline extract from cocoa husk may activate carbon-based materials as well good as more expensive imported analytically graded KOH for the synthesis of activated carbon for adsorption. This agrees with the work of Maliki et al.7 in which the alkaline extract from cocoa husk was used in hydrolysis and esterification reactions.
| Table 3: | Heavy metal contents of wastewater samples before and after adsorption | |||
After adsorption (Concentration mg L–1) |
|||||||||
Before adsorption (Concentration mg L–1) |
CAPAC | KPAC | |||||||
| Metals | EFF |
UPS |
DWS |
EFF |
UPS |
DWS |
EFF |
UPS |
DWS |
| Ca | 48.943 |
61.9145 |
66.0512 |
0.932 |
0.9684 |
0.9096 |
0.8808 |
0.771 |
0.6749 |
| Mn | 0.0327 |
0.033 |
0.0192 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Cu | 0.0198 |
0.0247 |
0.0197 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Cr | 0.0112 |
0.0175 |
0.018 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Cd | 0.0006 |
- |
- |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Zn | 0.0643 |
0.0594 |
0.043 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Na | 11.7382 |
11.8053 |
12.6143 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Fe | 0.1889 |
0.1649 |
0.01357 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Mg | 0.642 |
0.2096 |
0.2337 |
BDL |
0.0004 |
0 |
0.0001 |
0.00017 |
0.000172 |
| K | 4.6353 |
5.3708 |
5.7602 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| V | 0.013 |
0.0123 |
0.0142 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Hg | 0.0866 |
0.0868 |
0.0833 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Ti | 0.0049 |
0.0012 |
0.0012 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Ta | - |
- |
0.0004 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| S | 0.0572 |
0.2167 |
0.2691 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Si | 5.997 |
6.2037 |
7.2649 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| P | 0.1989 |
0.0075 |
- |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Nb | 0.0007 |
0.0006 |
0.0006 |
0.000337 |
0.0009 |
0.000036 |
0.000169 |
BDL |
0.000476 |
| B | 0.0159 |
0.0095 |
0.0082 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Sr | 0.1809 |
0.2409 |
0.2484 |
0.003 |
0.0003 |
0.0034 |
0.0031 |
0.0011 |
0.0009 |
| In | 11.7382 |
0 |
0.0032 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Li | 0.0029 |
0.0043 |
0.004 |
0.00008 |
BDL |
0.000108 |
BDL |
BDL |
BDL |
| Ba | 0.1905 |
0.1549 |
0.1531 |
0.048 |
0.042 |
0.0532 |
0.0414 |
0.0375 |
0.0402 |
| Ru | 0.002 |
0.0053 |
0.0051 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Pd | 0.0013 |
- |
0.0009 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| U | 0.0066 |
0.0075 |
0.0128 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Tm | 0.0004 |
0.0002 |
0.0001 |
0.00046 |
0.000244 |
0.000112 |
0.0000268 |
0.000137 |
0.00012 |
| Sc | 0.0001 |
- |
0.0001 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Lu | - |
0 |
0.0002 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| La | 0.0097 |
0.0105 |
0.0112 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Eu | 0.0013 |
0.0006 |
0.0007 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Ce | 0.0077 |
0.0069 |
0.0126 |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| Ag | 0.0002 |
- |
- |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
| CPAC: Cocoa-PET activated carbon, KPAC: KOH-PET activated carbon, BDL: Below detection limit, EFF: Effluent, UPS: Upstream, DWS: Downstream and Bold: Metals that are of concern due to their high concentrations | |||||||||
| Table 4: | Langmuir and freundlich constants for the adsorption of metals Ca, Cr, Zn, Mn, Cu, Fe utilizing CPAC and KPAC | |||
Langmuir constants |
Freundlich constants |
|||||
| Adsorbents | qm (mg/g) |
ka (L/mg) |
R2 |
I/n |
kf (mg/gL/mg)1/n) |
R2 |
| CPAC | ||||||
| Calcium | 20.161 |
0 |
-0.607 |
0.0911 |
1 |
0.7878 |
| Chromium | 10.977 |
0 |
0.4528 |
0.6909 |
1 |
0.6392 |
| Zinc | 21.65 |
0 |
-0.283 |
1.3077 |
1 |
0.7846 |
| Manganese | 0.0014 |
0 |
-1.715 |
1.0859 |
1 |
0.5513 |
| Copper | 0.0034 |
1 |
0.0221 |
0.21 |
1 |
-9.568 |
| Iron | 8×10-5 |
0 |
-0.096 |
0.8598 |
1 |
-2.00E+15 |
| KPAC | ||||||
| Calcium | 23.041 |
0.001 |
0.4667 |
0.102 |
1 |
0.6935 |
| Chromium | 9.804 |
0 |
0.5623 |
0.6998 |
1 |
0.6539 |
| Zinc | 0.0456 |
0 |
-0.578 |
1.3077 |
1 |
0.7846 |
| Manganese | 0.007 |
0 |
-1.202 |
1.152 |
1 |
0.8568 |
| Copper | 0.0003 |
0 |
-1.26 |
1.03 |
1 |
-0.331 |
| Iron | 0.0026 |
0 |
-0.955 |
0.8969 |
1 |
0.6923 |
| R2: Correlation coefficient, CPAC: Cocoa-PET activated carbon and KPAC: KOH-PET activated carbon | ||||||
Adsorption isotherms: Table 4 showed the outcomes of the sorption ability of CPAC and KPAC using the adsorption isotherms of the Ca, Zn, Cr, Mn, Cu and Fe sorption systems. The coefficients of these isotherms (Langmuir and Freundlich) were also presented in Table 4. The correlation coefficient R2 was higher in Freundlich isotherm than Langmuir isotherm. This suggests that the adsorption process works better with Freundlich isotherms than with Langmuir isotherms. Although some of the isotherms showed negative values for both isotherms. Both adsorbents exhibit high adsorption capacity (mg g–1) for calcium and chromium while CPAC revealed better adsorption capacity towards Zinc compared to KPAC.
CONCLUSION
The PET can be employed as a precursor for the manufacture of activated carbon, with alkaline filtrate from cocoa husk serving as the activating agent, according to the findings. The activated carbon formed could be used to remove metals from wastewater as an adsorbent. For Ca, Zn, Cr, Mn, Cu and Fe, the best adsorption conditions are 0.01 and 0.20 mg L–1 initial metal concentration, pH 10 and 12, 15 min contact duration and 0.25 g adsorbent dose, respectively. According to Langmuir and Freundlich adsorption isotherm models, CPAC and KPAC are effective for this adsorption. The PET and cocoa husk will then not be a nuisance to the environment but the solid waste generated, managed and processed into useful products. To enhance the national economy, small-scale business investors should prioritize bulk manufacture of activated carbon from polyethylene terephthalate and alkali extract from the cocoa husk. Further research is encouraged to be carried out on the use of these adsorbents in the treatment of surface and other contaminated water.
SIGNIFICANCE STATEMENT
The management and cleanup of the environment face significant challenges due to wastewater treatment. The methods that are available are time-consuming, expensive and unsustainable. Polyethylene terephthalate was suggested as an alternative due to the need to develop less time-consuming, environmentally friendly technologies, but its effectiveness in cleaning wastewater from cement factories has not been established, hence this study.
ACKNOWLEDGMENT
The authors gratefully acknowledge the members and volunteer employees of the SwWECh Laboratory and Research Unit (previously SwWECh laboratory), Institute of Ecology and Environmental Studies, Obafemi Awolowo University, Ile-Ife, Nigeria, for their invaluable contributions and for carrying out the majority of the field and laboratory analyses.
REFERENCES
- Daughton, C.G., 2004. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev., 24: 711-732.
- Du, B., A.E. Price, W.C. Scott, L.A. Kristofco, A.J. Ramirez et al., 2014. Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent. Sci. Total Environ., 466-467: 976-984.
- Zerrouqi, Z., M. Sbaa, M. Oujidi, M. Elkharmouz, S. Bengamra and A. Zerrouqi, 2008. Assessment of cement's dust impact on the soil using principal component analysis and GIS. Int. J. Environ. Sci. Technol., 5: 125-134.
- Olohigbe, A.B., O.R. Etiosa and O. Wesley, 2018. Highly deacetylated chitosan as low-cost adsorbent material for removal of heavy metals from water. Asian J. Phys. Chem. Sci., 5: 38599.
- Chaudhari, V. and M. Patkar, 2022. Removal of nickel from aqueous solution by using corncob as adsorbent. Mater. Today: Proc., 61: 307-314.
- Balakrishnan, B., D.S. Kumar, Y. Yoshida and A. Jayakrishnan 2005. Chemical modification of poly(vinyl chloride) resin using poly(ethylene glycol) to improve blood compatibility. Biomaterials, 26: 3495-3502.
- Maliki, M., I.H. Ifijen and S.O. Omorogbe, 2020. Cocoa husks: A sustainable resource for alkali production. Int. J. Bio. Chem. Sci., 14: 2652-2658.
- Okoya, A.A., A.B. Akinyele, I.E. Ofoezie, O.S. Amuda, O.S. Alayande and O.W. Makinde, 2014. Adsorption of heavy metal ions onto chitosan grafted cocoa husk char. Afr. J. Pure Appl. Chem., 8: 147-161.
- Sabir, A., F. Altaf, R. Batool, M. Shafiq, R.U. Khan and K.I. Jacob, 2021. Agricultural Waste Absorbents for Heavy Metal Removal. In: Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water, Inamuddin, M.I. Ahamed, E. Lichtfouse and A.M. Asiri (Eds.), Springer, Cham, Switzerland, ISBN: 978-3-030-47400-3, pp: 195-228.
- Okoya, A.A., O.O. Olaiya, A.B. Akinyele and N.O. Ochor, 2020. Efficacy of Moringa oleifera seed husk as adsorptive agent for trihalomethanes from a water treatment plant in Southwestern, Nigeria. J. Chem., 2020.
- Kadirvelu, K., K. Thamaraiselvi and C. Namasivayam, 2001. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol., 76: 63-65.
- Newcombe, G., 2006. Removal of Natural Organic Material and Algal Metabolites Using Activated Carbon. In: Interface Science and Technology. Newcombe, G. and D. Dixon (Eds.), Elsevier, Amsterdam, Netherlands, ISBN: 9780120883806, pp: 133-153.
- Alalwan, H.A., M.A. Kadhom and A.H. Alminshid, 2020. Removal of heavy metals from wastewater using agricultural byproducts. J. Water Supply: Res. Technol. Aqua, 69: 99-112.
- Okoya, A.A., A.B. Akinyele, O.S. Amuda and I.E. Ofoezie, 2016. Chitosan-grafted carbon for the sequestration of heavy metals in aqueous solution. Chem. Sci. Int. J., 11: 21813.
- Haruna, S.A., A. Musa and G.T. Ayinla, 2020. Trace and heavy metal contamination status of soil and water in artisanal and small scale gold mining vicinity in Kuchiko-Hausa, Gurara LGA, Niger State, Nigeria. Earthline J. Chem. Sci., 5: 207-219.
- Bhatti, H.N., A.W. Nasir and M.A. Hanif, 2010. Efficacy of Daucus carota L. waste biomass for the removal of chromium from aqueous solutions. Desalination, 253: 78-87.
- Ali, I., 2012. New generation adsorbents for water treatment. Chem. Rev., 112: 5073-5091.
- Mendoza-Carrasco, R., E.M. Cuerda-Correa, M.F. Alexandre-Franco and C. Fernández-González and V. Gómez-Serrano, 2016. Preparation of high-quality activated carbon from polyethyleneterephthalate (PET) bottle waste. Its use in the removal of pollutants in aqueous solution. J. Environ. Manage., 181: 522-535.
- de Castro, C.S., L.N. Viau, J.T. Andrade, T.A.P. Mendonça and M. Gonçalves, 2018. Mesoporous activated carbon from polyethyleneterephthalate (PET) waste: Pollutant adsorption in aqueous solution. New J. Chem., 42: 14612-14619.
- Ahangar, F.A., U. Rashid, J. Ahmad, T. Tsubota and A. Alsalme, 2021. Conversion of waste polyethylene terephthalate (pet) polymer into activated carbon and its feasibility to produce green fuel. Polymers, 13: 3952.
- Marsh, H. and F. Rodriguez-Reinoso, 2006. Activated Carbon. 1st Edn., Elsevier, Amsterdam, Pages: 536.
- Renu, M. Agarwal and K. Singh, 2017. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin., 7: 387-419.
- Alwaan, I.M. and M.A.K. Jaleel, 2021. Preparation and characterization of activated carbon with (ZnCl2-activated) from (pet) bottle waste for removal of metal ions (Cu+2) in aqueous solution. IOP Conf. Ser.: Mater. Sci. Eng., 1094.
- Malik, P.K., 2003. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of acid yellow 36. Dyes Pigm., 56: 239-249.
- Velasquez, L. and J. Dussan, 2009. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J. Hazard. Mater., 167: 713-716.
- Lukman, S., M.H. Essa, N.D. Mu`azu, A. Bukhari and C. Basheer, 2013. Adsorption and desorption of heavy metals onto natural clay material: Influence of initial pH. J. Environ. Sci. Technol., 6: 1-15.
- Zhang, Y., H. Zhang, Z. Zhang, C. Liu, C. Sun, W. Zhang and T. Marhaba, 2018. pH effect on heavy metal release from a polluted sediment. J. Chem. 2018: 7597640.
- Sugashini, S. and S. Gopalakrishnan, 2012. Studies on the performance of protonated cross linked chitosan beads (PCCB) for chromium removal. Res. J. Chem. Sci., 2: 55-59.
- Bernard E., A. Jimoh and J.O. Odigure, 2013. Heavy metals removal from industrial wastewater by activated carbon prepared from coconut shell. Res. J. Chem. Sci., 3: 3-9.
How to Cite this paper?
APA-7 Style
Okoya,
A.A., Agbagu,
V.D., Akinyele,
A.B. (2023). Sorption of Selected Metals from Cement Factory Wastewater using Alkali Activated Carbons Derived from PET (Polyethylene terephthalate). Trends in Applied Sciences Research, 18(1), 103-117. https://doi.org/10.3923/tasr.2023.103.117
ACS Style
Okoya,
A.A.; Agbagu,
V.D.; Akinyele,
A.B. Sorption of Selected Metals from Cement Factory Wastewater using Alkali Activated Carbons Derived from PET (Polyethylene terephthalate). Trends Appl. Sci. Res 2023, 18, 103-117. https://doi.org/10.3923/tasr.2023.103.117
AMA Style
Okoya
AA, Agbagu
VD, Akinyele
AB. Sorption of Selected Metals from Cement Factory Wastewater using Alkali Activated Carbons Derived from PET (Polyethylene terephthalate). Trends in Applied Sciences Research. 2023; 18(1): 103-117. https://doi.org/10.3923/tasr.2023.103.117
Chicago/Turabian Style
Okoya, Aderonke, Adetutu, Victor Damilola Agbagu, and Abimbola Bankole Akinyele.
2023. "Sorption of Selected Metals from Cement Factory Wastewater using Alkali Activated Carbons Derived from PET (Polyethylene terephthalate)" Trends in Applied Sciences Research 18, no. 1: 103-117. https://doi.org/10.3923/tasr.2023.103.117

This work is licensed under a Creative Commons Attribution 4.0 International License.



