Hazard Indices and Human Health Risks Associated with PAHs Exposure from Consumption of Bivalve Shellfish from Niger Delta, Nigeria
| Received 18 Dec, 2021 |
Accepted 08 Aug, 2022 |
Published 31 Mar, 2023 |
Background and Objective: Polycyclic Aromatic Hydrocarbon (PAHs) remains a potential food safety hazard associated with seafood harvested from the brackish waters of the Niger Delta. This is attributable to the high level of environmental degradation posed by petroleum production and exploitation along the coastline. This study investigated hazard indices and human health risks associated with Polycyclic Aromatic Hydrocarbon (PAHs) exposure from consumption of bivalve shellfish from the Niger Delta. Materials and Methods: Four samples of bivalve shellfish: Bloody cockle (Anadara senilis), Donax clam (Donax rugosus), Knife clam (Tagelus adansonaii) and Mangrove oyster (Crassostrea gasar) harvested from four locations in the Niger Delta were assessed for PAHs concentrations through the use of Gas chromatography. The United State Environmental Protection Agency method was used to estimate the human health risk of PAHs in bivalves consumed in the Niger Delta. Results: The result indicated an elevated tissue concentration of polycyclic aromatic hydrocarbons in bivalve shellfish above the legal limits allowed for food safety. The estimated values for human health risk assessment revealed a non-carcinogenic value and hazard index less than one for non-carcinogenic PAHs while risk value for carcinogenic PAHs and cumulative cancer indices at some study locations were higher than the stipulated one in one million (1.0×10‾6) chances as stipulated by regulatory bodies which imply that carcinogenic effects were more likely due to consumption of 48 g day‾1 of bivalve shellfish with PAHs contaminants. Conclusion: Therefore consumers are likely to experience significant health risks through the consumption of bivalve shellfish from the study locations.
| Copyright © 2023 Ukwo et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Polycyclic Aromatic Hydrocarbons (PAHs) consists of a group of complex environmental contaminants that are common in several ecological matrices such as water, air, soil and even dust. They have the capabilities of potential accumulation by some environmental biota. They constitute persistent environmental contaminants that may originate from anthropogenic activities such as incomplete combustion and pyrolysis processes of organic substances, gas flaring and domestic/municipal incineration as well as from natural sources1. They are lipophilic compounds that consist of two or more benzene rings fused together2. The PAHs occur naturally as constituents of crude oil, coal, bitumen and natural gas. The PAHs exist as solid and colourless substances free from contaminants if intended for use in research purposes3. The PAHs with more than four benzene rings are classified as High Molecular Weights (HMW), which are considered more carcinogenic and genotoxic compared to those of Low Molecular Weight (LMW) which are sometimes not defined as carcinogenic but may act as synergists4. According to Brown et al.5, more than 100 individual PAHs exist but 16 priority PAHs have been earmarked for global monitoring3 and are of focus in several ecological studies. Of these priority PAHs, benzo(a)pyrene is considered as the most potent and well reported as the bio-indicator of PAHs (toxicity) in the reviewed papers, with a maximum European Union (EU) allowable limit of 2 μg kg–1 in fish4. The use of benzo(a)pyrene as a biomarker in seafood was stipulated by the EU regulation adopted by member countries. The input sources of PAHs from petroleum (petrogenic) or combustion (pyrogenic) into the environment or biological sample can be determined according to Beg et al.3 by finding the ratio of LMW: HMW. A diagnostic ratio of LMW: HMW >1 implies petrogenic (petroleum), while LMW: HMW <1 implies pyrogenic.
Bivalve shellfish are soft-bodied invertebrates that are enclosed in a hard shell6. Bivalve shellfish inhabit fresh and marine waters from the abyssal depths of high intertidal areas in tropical to warm temperate waters. They are considered delicious and healthy food items in several dietary regimes in different parts of the world. The most important species are the clams, mussels, oysters and scallops7. The pattern of their distribution is characteristically different from one location to another depending on the sediment types, variations in water salinities and tidal movements8.
Bivalve mollusc shellfish is a veritable source of protein with good quality amino acids. They are also rich in essential and trace mineral elements such as calcium, magnesium zinc iron and phosphorus as well as vitamins A, B and E9. The nutritional content of bivalve shellfish differs from one species to the other and with the same species. Factors such as environment, age, feed composition, degree of maturation, seasonal changes and sex have direct effects on the nutritional quality of bivalve10. Despite the numerous advantages of a seafood-based diet, adverse health effects can also exist and seafood harvested from polluted aquatic environments can contain biological and chemical contaminants11. Bivalves shellfish being sedimentary filter feeders feed by opening their shells for absorption food particles. Due to their feeding pattern, they filter tiny particles of aquatic plants, animals and inorganic matter. It also accumulates the diversities of other contaminants from the surrounding seawater12,13. These feeding patterns render the bivalve shellfish easily prone to bacteria, heavy metals, biotoxins and other environmental contaminations8. Most species of bivalve shellfish consumed in Nigeria are harvested from the brackish water that is exposed to varying amounts of chemical and environmental contaminants such as industrial chemicals, toxic residues from various anthropogenic activities.
Artisanal refining of petroleum products, spillage and leakage of oil, gas flaring and well bush burning in the Niger Delta Region are prevalent with its attending consequences. Presently, several oilfields are encompassing onshore and offshore facilities14. According to the reports of Amnesty International15, about 179 sites flare gas in the Niger Delta Region, which cumulatively releases over a billion standard cubic feet of gas with flaring points located at the offshore production sites. Such a high quantum gas emitted into the atmosphere have given rise to higher temperatures which rendered vast areas of land uninhabitable16. Also, recurrent explosion and leakages from refined products, oil and gas pipelines, as well as vandalism and illegal mining activities in this area, have resulted in the burning of viable mangrove habitat and vegetation, A reports from United Nations Development Programme17 on the Niger Delta pollution index estimated that about 89% of the gas production in the Niger Delta are flared into the environment with no concomitant or remarkably efforts at mitigating the impact to the environment and other ecological receptors. Therefore with the accumulative nature of the released pollutant, the hydrocarbon pollution index is expected to be significantly alarming and much higher than the acceptable threshold. Bivalve including mangrove oysters, bloody cockle, clams among others are suspension or filter feeders that take in chemical contaminants and microbes from the polluted Niger Delta waters can accumulate in their tissue posing serious concern in their quality and safety. Therefore, the continuous consumption of bivalve shellfish from the Niger Delta waters posits or exemplifies the conflict between food benefits and food risks. The international law and other charters encouraged the State and stakeholders to improve upon, protect and provide its citizens with different sources of food. The major food sources should therefore be seriously protected, not abused or contaminated by private individuals or organizations thereby preventing peoples’ ability at feeding themselves. The multidisciplinary and multi-sectoral approach to the sustainable mitigation of health risks resulting from pollution in this region is paramount and the assessment of levels of polycyclic aromatic hydrocarbons in bivalve shellfish in the Niger Delta and the associated human health risks resulting from the consumption of bivalve shellfish offer a valuable strategy. The objectives of this study were to assess the source and levels of polycyclic aromatic hydrocarbons accumulated by bivalve shellfish as well as the associated human health risk associated with their consumption. It is hoped that this study will expose the suitability of these benthic organisms for food as well as serve as an ideal indicator of biotic integrity therefore their studies are a direct assessment of the environmental health of landscapes that drain into them since rivers are at the receiving end of pollution effect of the estuarine and the coastal waters of this region.
MATERIALS AND METHODS
Study location: The study location lies along the Atlantic coastline in the Niger Delta Region of Nigeria from February, 2017 to September, 2021. Four locations were chosen for this study (Andoni, Bonny, Ibeno and Iko Town). The locations were chosen because of their popularity in artisanal fishing activities particularly on bivalve shellfish which also served as an important delicacy and food for the locales. Shellfish also served as a major source of income and employment for the people in these communities. The locations are essentially estuarine with brackish water characterized by fine sandy beaches surrounded with mangrove swamp and intertidal mudflat in which Nypa vegetation dominate. The area is also naturally endowed with an abundance of rivers, creeks and streams which received water and waste from the hinterland into the Atlantic Ocean. Also, this coastal environment has continued to suffer from environmental degradation occasioned by exploration and production of petroleum, liquefied natural gas production and spillage of petroleum products.
Sample preparation and treatments: Samples of bivalve shellfish mostly consumed in these localities were harvested manually by fishermen during low tide from intertidal estuarine mudflats of the different study locations. The bivalve specimens collected were, Bloody cockle Anadara senilis, Donax clam Donax rugosus, Knife or Razor clam Tagelus adansonaii and Mangrove oyster Crassostrea gasar. They were identified at the Department of Fisheries and Aquatic Environmental Management, University of Uyo. At each sampling site, twenty samples of each bivalve specimen were collected and transferred to the laboratory within 24 hrs of collection in plastic containers washed with 5% nitric acid and rinsed with distilled water before use. At the laboratory, the bivalves were promptly cleaned of incrustations, washed in distilled water to remove all dirt. Samples were shucked with the sterile scalpel to extract the flesh and intravalvular fluid into a sterile container. The extracted tissues were homogenized for the 60 s in a stomacher (Seward Laboratory Stomacher 400, England) and stored at -20°C in a scan frost deep freezer for PAHs analysis.
Determination of concentration of polycyclic aromatic hydrocarbons in bivalve samples: The target organic contaminants in this study were the 16 US EPA Polycyclic Aromatic Hydrocarbons (PAHs) earmarked for global monitoring5. The determination of 16 priority polycyclic aromatic hydrocarbons (PAHs) was carried out through extraction, sample clean-up, pre-concentration or sample enrichment and instrumental analysis. Before extraction, the dried bivalve samples were ground to powder using pestle and mortar to ensure homogenization. The powdered samples were then Soxhlet extracted according to the USEPA guideline, as described by Cheung et al.18. Analysis of (PAHs) was done using an Agilent 6890N gas chromatography with flame ionization detector (GC/FID), equipped with an injector fused with a silica capillary column (DB-5 ms). Helium gas was used as the carrier with a split ratio of 50:1 and the temperature of 300°C was maintained as set point Quality control measures included the use of high purity (95-99.9%) external standard mixture. Analytical grade solvents and reagents (acetone, hexane, dichloromethane and anhydrous sodium sulphate) obtained from Merck (USA) were used for the extraction. Standard and surrogate solutions were prepared in hexane. To correct for PAH recovery efficiencies, bivalve samples were spiked with 1ml of a solution of five internal standards (biphenyl-d, anthracene-d, phenanthrene-d, pyrene-d10, perylene-d) each at 2 μg mL–1 in hexane.
Human health risk assessment procedure: Assessment of human health risk for ingesting bivalve shellfish with, polycyclic aromatic hydrocarbons contaminants were determined based on methods US EPA, as described by Cheung et al.18. The data collected from this study was used. The following section explains the various equations used to determine risk for non-carcinogens and carcinogens in this study.
Determination of estimated daily intake: The level of exposure resulting from consumption of a particular chemical in bivalve samples can be expressed by estimating the daily intake levels in the following equation below:
| EDI | = |
Estimated Daily Intake (mg kg–1 day–1) |
| IR | = |
Ingestion rate of Bivalve (kg day–1) |
| C | = |
Concentration of chemical contaminant in bivalve tissue (mg kg–1) |
| EF | = |
Exposure frequency (days/year) |
| ED | = |
Exposure duration (years) |
| BW | = |
Body weight (kg) |
| AT | = |
Average time (days) |
Risk for both carcinogenic and non-carcinogenic chemicals was calculated in this study and the above equation was used as the intake equation.
Calculation of Non-carcinogenic Risk or Target Hazard Quotient (THQ): The non-carcinogenic risk due to consumption of chemical contaminants was determined using Target Hazard Quotient values (THQ). It is the ratio between the exposure rate and the reference dose. The calculations were made using the standard assumption for an integrated USEPA risk analysis.
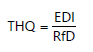 |
THQ |
= |
Non-carcinogenic risk value of chemicals contaminants |
EDI |
= |
Estimated daily intake or exposure rate (mg/kg/day) |
RfD |
= |
Reference dose of chemical (mg/kg/day) |
When the intake or average daily dose is equal to the reference dose, the non-carcinogenic value or THQ is one. When the Estimated Daily Intake (EDI) exceeds the reference dose and thus, the THQ is greater than one or equal to one, it is the likelihood that non-carcinogenic adverse health effects will be observed due to ingestion of 48 g of bivalve sample per day but when THQ is less than one its indicate that adverse health effects are not likely to occur due to ingestion of 48 g of bivalve sample per day. For this study, the Intake Rate of fish (IR) was assumed to be 48 g/person per day12, the average body weight of exposed individual (70 kg), exposure frequency (365 day/year) while duration of exposure was taken to be an average life expectancy of a Nigerian (55.20 years) as reported by WHO12. The length of time for the average dose was calculated as 365 days×55.20 years. It was also assumed that ingested doses were equal to absorbed contaminants doses. The Hazard Index (HI) is the sum of total hazard quotients, (HI = ΣTHQ). The values for non-carcinogenic risk of individual PAHs for this study were calculated using their Respective Reference Doses (RfD) in mg/kg/day as listed by USEPA5:
| Nap | = |
2.0×10–2 |
| Ace | = |
6.0×10–2 |
| Acy | = |
6.0×10–2 |
| Flu | = |
4.0×10–2 |
| Ant | = |
3.0×10–1 |
| Phe | = |
7.1×10–3 |
| Fluo | = |
4.0×10–2 |
| Pyr | = |
3.0×10–2 |
| Bghi P | = |
3.0×10–2 |
Estimation of carcinogenic risk: Carcinogens are assumed to not have an effective or safe threshold. Carcinogenic risk is expressed as a product of EDI and cancer potency value or Cancer Slope Factor (CSf) and the following equation was used to estimate the lifetime risk of cancer:
Risk |
= |
Life time cancer risk |
EDI |
= |
Estimated Daily Intake mg/kg/day |
Csf |
= |
Cancer slope factor mg/kg/day |
USEPA21 guidelines on Integrated Risk Information System (IRIS) specify that acceptable risk is a lifetime cancer risk not greater than 1 in 106 (one in one million chance). The carcinogenic risk value for inorganic arsenic was determined using the cancer slope factor (CSf) of 1.5 mg/kg/day. The risk indices for carcinogenic PAHs were determined using benzo(a)pyrene Cancer Slope Factor (CSf) value of 7.39 mg/kg/day.
Experimental design and data analysis: A two factor (4x4) factorial experiment with location and species of bivalve samples being factor A and B respectively was used to study the compositional pattern and tissue concentration of Polycyclic aromatic hydrocarbons in the Niger Delta. Data obtained from analyses were subjected to a two-way Analysis of Variance (ANOVA) to evaluate the effect of location and species on bivalve molluscs. The level of significance was set at p<0.05. Means with significant differences were separated using Ducan-Multiple Range Test. All experiments were conducted in triplicate and data were analysed using XLSTAT-Pro software program, Addinsoft, Boston (USA) Version 2018.7.
RESULTS AND DISCUSSION
Bioaccumulation of polycyclic aromatic hydrocarbons by bivalve shellfish: The levels of Polycyclic Aromatic Hydrocarbons (PAHs) accumulated by each species of bivalve shellfish is shown in Table 1. The result indicated that bloody cockle at Andoni had the highest PAH concentration of 83.19 μg kg–1 followed by Knife clam 76.47 μg kg–1 at the same location while the least was in Knife clam with 14.51 μg kg–1 at the Ibeno location. Also, samples from Andoni were dominated with LMW-PAHs when compared to samples from Ibeno and Iko Town whose samples were dominated by HMW-PAHs. The diagnostic ratio for the interaction was a mixed one which indicated a petrogenic source in Andoni samples except for bloody cockle while all the samples from Ibeno and Iko Town were from pyrogenic source19. Results obtained from the interaction of species and location also showed variability in tissue concentration concerning location. Bloody cockle had the highest tissue concentration at Andoni and Bonny while mangrove oysters had the highest concentration at Ibeno and Iko Town locations. The total concentration of PAH concentration (μg kg–1) in bivalve samples from the study locations also indicated a higher accumulation in Andoni and Bonny locations when compared with the bivalve samples harvested from the coastal waters of Ibeno and Iko Town. The LMW-PAHs are generally not considered to have health impacts on people at environmental exposures levels. However, the duration of oral could determine the kind of resultant risk experienced by a human for instance short-term exposure to LMW PAHs can result in tissue damage and inflammation of vital organs in the body and gastrointestinal damage20 while protracted exposures can result in renal damage, haematological effects and reproductive impairment in animals. In addition to irritation, decreased fertility, developmental neurological effects and liver toxicity have been proven in laboratory animals exposed to relatively higher concentrations of PAHs. Research has proven most that long term PAH exposure results from cancer21. Also, synergistic effects of HMW-PAHs congeners have been shown to have different cancer potencies which may induce different types of cancer in laboratory experiments for instance in oral exposure to benzo(a)pyrene or dibenzo(a,h)pyrene predominantly resulted in gastrointestinal tract cancers or lung cancer, respectively22. Research has shown that toxicity and persistence of PAHs increases with an increase in the number of rings. The four rings fused PAHs such as benzo(a)anthracene and chrysene, are not so much carcinogenic and persistent. The five or six-fused ring PAHs, such as benzo(b)fluoranthene, benzo(a)pyrene and indo(1,2,3-cd)pyrene and benzo(g,h,i)perylene are very potent carcinogens and also persist23,24.
Risk assessment of PAHs from consumption of bivalve samples
Non-carcinogenic risks of PAHs: The results of the determination of hazard indices (HI) and non-carcinogenic risk values of non-carcinogenic PAHs in bivalve shellfish harvested from the coastal waters of the Niger Delta is presented in Table 2. Values for naphthalene ranged from 3.43×10–8-2.42×10–4. Donax clam from Andoni had the highest value (2.42×10–4) for naphthalene (Nap) non-carcinogenic value for acenapthylene (Acy) and acenaphthene (Ace), respectively. The non-carcinogenic risk for fluorene ranged from 1.71×10–8-1.98×10–4, anthracene (Ant) 2.29×10–9-3.51×10–5, phenanthrene (Phe) 9.66×10–8-1.09×10–3 and fluoranthene (Fluo) 171×10–8-2.21×10–4. The value for non-carcinogenic risk of pyrene (Pyr) ranged from 2.29×10–8-1.16×10–4 of which Donax clam from Ibeno and mangrove oyster from Ibeno had the lowest and highest values, respectively. Value for benzo(ghi)perylene ranged from 2.29×10–8-1.43×10–4. The hazard index (HI) for non-carcinogenic PAHs ranged from 9.22×10–5-1.66×10–3. Knife clam from Andoni had the highest hazard index while knife clam from the Ibeno location had the lowest hazard index. The non-carcinogenic risk value also referred to as Target Hazard Quotient (THQ) is an expression of the non-carcinogenic effect which is calculated based on Reference Dose (RfD) for the non-carcinogenic PAH congeners. A hazard index (HI) is the total chronic hazard attributable to exposure to all non-carcinogenic PAHs through the consumption of bivalve molluscs from the studied locations. It is calculated by summation of the non-carcinogenic risk value for each species. From the result of this study, the calculated THQ for all bivalve species from the study locations were less than one. This implies that the consumption of 48 g/day of any of the bivalve species from the study locations is not likely to cause any non-carcinogenic effects on consumers. The results from this study also showed that the calculated Hazard Index (HI) was less than one. Thus, the exposure to complex or cumulated PAHs would have little or no potential adverse effects on the local consumers. The result obtained from this study is in agreement with values reported by Mahammed et al.25 on cancer and non-cancer risk associated with PAHs exposure from fish in Northern Nigeria and Tongo et al.26, on human health risk assessment of PAHs in fish and shellfish from Amariaria Community, Bonny River, in the Niger Delta where the author noted no potential negative health risk of non-carcinogenic PAHs.
| Table 1: | Bioaccumulation of PAHs by bivalve shellfish (μg kg–1) | |||
Andoni |
Bonny |
Ibeno |
Iko Town |
|||||||||||||
| PAHs | Cockle |
Donax clam |
Knife clam |
Oyster |
Cockle |
Donax clam |
Knife clam |
Oyster |
Cockle |
Donax clam |
Knife clam |
Oyster |
Cockle |
Donax clam |
Knife clam |
Oyster |
| NaP | ND |
7.07a |
ND |
2.34b |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
| Acy | 11.41c |
2.94e |
12.68a |
4.65d |
2.11f |
ND |
ND |
12.34b |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
| Ace | ND |
0.75f |
1.65d |
2.72b |
1.44de |
1.13e |
11.40a |
2.27c |
ND |
ND |
1.10e |
2.25c |
0.18g |
ND |
0.54f |
ND |
| Flu | 9.35b |
1.53de |
3.47c |
1.14e |
11.18a |
11.27a |
11.56a |
11.40a |
ND |
ND |
0.26f |
3.29c |
1.84d |
ND |
1.19e |
ND |
| Ant | 15.34a |
11.58c |
13.53b |
1.35ef |
ND |
1.05f |
1.30ef |
1.18ef |
1.21ef |
ND |
ND |
2.60d |
0.86f |
1.63e |
2.34d |
2.80d |
| Phe | ND |
5.34b |
11.31a |
ND |
ND |
ND |
1.18d |
ND |
1.33c |
ND |
ND |
5.43b |
0.17f |
ND |
ND |
0.45e |
| Total LMW | 36.09 |
29.2 |
42.64 |
12.2 |
14.73 |
13.45 |
25.44 |
27.19 |
2.55 |
ND |
1.36 |
13.57 |
3.06 |
1.63 |
4.08 |
3.25 |
| Fluo | 12.91a |
1.71bc |
0.29cd |
1.24bcd |
1.23bcd |
1.20bcd |
1.11bcd |
ND |
2.25b |
2.44b |
2.12b |
2.17b |
0.34cd |
1.58bc |
0.42cd |
0.51cd |
| Pyr | 2.20cd |
1.27gh |
0.16i |
ND |
2.27c |
1.44efg |
1.84de |
1.24gh |
0.35i |
5.06a |
1.69ef |
4.14b |
0.94h |
1.40fg |
2.41c |
1.48efg |
| BaA | 2.03c |
ND |
ND |
1.74d |
ND |
ND |
ND |
ND |
ND |
1.46e |
ND |
2.17bc |
2.24b |
0.47f |
ND |
6.47a |
| Chr | ND |
0.60e |
ND |
ND |
11.29a |
ND |
ND |
1.21d |
1.63c |
ND |
ND |
0.42e |
ND |
ND |
1.61c |
2.46b |
| BbF | 1.61gh |
1.57gh |
2.11gh |
ND |
13.07de |
12.82de |
12.36e |
10.77f |
2.36g |
1.31h |
2.35g |
2.57g |
24.52a |
14.61c |
22.25b |
13.34d |
| BkF | 3.24b |
ND |
1.48e |
ND |
ND |
ND |
ND |
ND |
0.39f |
4.50a |
1.52e |
ND |
1.66d |
1.71d |
2.17c |
ND |
| BaP | 2.03cde |
2.06cde |
3.89b |
1.79de |
2.62c |
ND |
1.66ef |
1.14f |
2.44c |
2.20cde |
2.38cd |
4.64a |
1.78de |
0.51g |
1.64ef |
1.79de |
| BghiP | ND |
2.26g |
13.22b |
1.71i |
13.5a |
3.36e |
2.26g |
1.24j |
2.51f |
ND |
ND |
6.26d |
2.64f |
1.95h |
1.86h |
8.71c |
| DahP | 11.90c |
1.49h |
12.69b |
1.87g |
ND |
12.70b |
13.19a |
2.05fg |
3.21e |
1.21h |
ND |
5.39d |
2.24f |
1.85g |
ND |
1.94fg |
| Inp | 11.18c |
1.35i |
ND |
1.80h |
12.67b |
13.09a |
12.32b |
5.83d |
1.31i |
2.44g |
3.08f |
3.26f |
1.94h |
1.83h |
2.40g |
3.82e |
| Total HMW | 47.1 |
12.31 |
33.83 |
10.15 |
56.73 |
44.62 |
44.73 |
23.47 |
16.45 |
20.62 |
13.15 |
31.02 |
38.3 |
25.92 |
34.76 |
40.51 |
| ∑PAHs | 83.19 |
41.51 |
76.47 |
22.35 |
71.46 |
58.07 |
70.17 |
50.66 |
19 |
20.62 |
14.51 |
44.59 |
41.36 |
27.55 |
38.84 |
43.76 |
| LMW:HMW | 0.77 |
2.37 |
1.26 |
1.2 |
0.26 |
0.3 |
0.57 |
1.16 |
0.15 |
0 |
0.1 |
0.44 |
0.08 |
0.06 |
0.12 |
0.08 |
| Superscript alphabets showed different significance level | ||||||||||||||||
| Table 2: | Non-carcinogenic risk value for PAHs during consumption of bivalve samples | |||
| Location | Species | NaP |
Acy |
Ace |
Flu |
Ant |
Phe |
Fluo |
Pyr |
BghiP |
HI |
| Andoni | Bloody Cockle | 3.43E-08 |
1.30E-04 |
1.14E-08 |
1.60E-04 |
3.51E-05 |
9.66E-08 |
2.21E-04 |
5.04E-05 |
2.29E-08 |
5.97E-04 |
| Donax clam | 2.42E-04 |
3.36E-05 |
8.53E-06 |
2.62E-05 |
2.65E-05 |
5.16E-04 |
2.93E-05 |
2.90E-05 |
5.17E-05 |
9.63E-04 |
|
| Knife clam | 3.43E-08 |
1.45E-04 |
1.89E-05 |
5.95E-05 |
3.09E-05 |
1.09E-03 |
4.91E-06 |
3.58E-06 |
3.02E-04 |
1.66E-03 |
|
| Mangrove oyster | 8.01E-05 |
5.31E-05 |
3.10E-05 |
1.95E-05 |
3.09E-06 |
9.66E-08 |
2.13E-05 |
2.29E-08 |
3.92E-05 |
2.47E-04 |
|
| Bonny | Bloody Cockle | 3.43E-08 |
2.42E-05 |
1.64E-05 |
1.92E-04 |
2.29E-09 |
9.66E-08 |
2.10E-05 |
5.19E-05 |
3.10E-04 |
6.16E-04 |
| Donax clam | 3.43E-08 |
1.14E-08 |
1.29E-05 |
1.93E-04 |
2.40E-06 |
9.66E-08 |
2.06E-05 |
3.28E-05 |
7.69E-05 |
3.39E-04 |
|
| Knife clam | 3.43E-08 |
1.14E-08 |
1.30E-04 |
1.98E-04 |
2.97E-06 |
1.14E-04 |
1.90E-05 |
4.20E-05 |
5.16E-05 |
5.58E-04 |
|
| Mangrove oyster | 3.43E-08 |
1.41E-04 |
2.59E-05 |
1.95E-04 |
2.69E-06 |
9.66E-08 |
1.71E-08 |
2.83E-05 |
2.83E-05 |
4.22E-04 |
|
| Ibeno | Bloody Cockle | 3.43E-08 |
1.14E-08 |
1.14E-08 |
1.71E-08 |
2.77E-06 |
1.29E-04 |
3.85E-05 |
8.00E-06 |
5.74E-05 |
2.36E-04 |
| Donax clam | 3.43E-08 |
1.14E-08 |
1.14E-08 |
1.71E-08 |
2.29E-09 |
9.66E-08 |
4.19E-05 |
1.16E-04 |
2.29E-08 |
1.58E-04 |
|
| Knife clam | 3.43E-08 |
1.14E-08 |
1.26E-05 |
4.40E-06 |
2.29E-09 |
9.66E-08 |
3.63E-05 |
3.87E-05 |
2.29E-08 |
9.22E-05 |
|
| Mangrove oyster | 3.43E-08 |
1.14E-08 |
2.57E-05 |
5.65E-05 |
5.94E-06 |
5.24E-04 |
3.72E-05 |
9.46E-05 |
1.43E-04 |
8.87E-04 |
|
| Iko Town | Bloody Cockle | 3.43E-08 |
1.14E-08 |
2.10E-06 |
3.15E-05 |
1.97E-06 |
1.67E-05 |
5.83E-06 |
2.16E-05 |
6.04E-05 |
1.40E-04 |
| Donax clam | 3.43E-08 |
1.14E-08 |
1.14E-08 |
1.71E-08 |
3.72E-06 |
9.66E-08 |
2.71E-05 |
3.19E-05 |
4.46E-05 |
1.08E-04 |
|
| Knife clam | 3.43E-08 |
1.14E-08 |
6.21E-06 |
2.05E-05 |
5.36E-06 |
9.66E-08 |
7.20E-06 |
5.52E-05 |
4.25E-05 |
1.37E-04 |
|
| Mangrove oyster | 3.43E-08 |
1.14E-08 |
1.14E-08 |
1.71E-08 |
6.40E-06 |
4.38E-05 |
8.74E-06 |
3.38E-05 |
1.99E-04 |
2.92E-04 |
|
| Nap: Naphthalene, Acy: Acenapthylene, Ace: Acenaphthene, Flu: Fluorene, Phen: Phenanthrene, Anth: Anthracene, Fluo: Fluoranthene, Py: Pyrene and BghiP: Benzo(g,h,i)perylene | |||||||||||
Carcinogenic Health Risk of PAHs: The results of the determination of carcinogenic risk as shown in Table 3 indicated risk indices for benzo(a)anthracene (BaA) ranged from 5.07×10–9-1.13×10–5 chrysene (Chr) ranged from 5.07×10–9-5.72×10–5, benzo(b)fluoranthene (BbF) ranged from 5.07×10–9-1.13×10–4 benzo(k)fluoranthene (BkF) ranged from 5.07×10–9-2.28×10–5, benzo(a)pyene (BaP) ranged from 5.07×10–9-2.35×10–5, dibenzo(a,h) anthracene (DahA) range from 5.07×10–9-6.68×10–5 and indo (1,2,3-cd) pyrene (InP) ranged from 5.07×10–9-6.63×10–5. The cancer risk values of some PAHs were observed to be higher than the acceptable risk level. The lifetime carcinogenic risk (LCR) values for benzo(a)anthracene in bloody cockle harvested from at Andoni (1.03×10–5), Iko Town (1.13×10–5) and mangrove oyster from Ibeno (1.10×10–5) and Iko Town (3.28×10–5) were higher than one in one million chance proposed by USEPA18.
| Table 3: | Carcinogenic risk value for PAH during consumption of bivalve samples | |||
| Location | Species | BaA |
Chr |
BbF |
BKF |
BaP |
DahP |
Inp |
∑LCR |
| Andoni | Bloody Cockle | 1.03E-05 |
5.07E-09 |
8.14E-06 |
1.64E-05 |
1.03E-05 |
6.03E-05 |
5.67E-05 |
1.62E-04 |
| Donax clam | 5.07E-09 |
3.04E-06 |
7.96E-06 |
5.07E-09 |
1.05E-05 |
7.57E-06 |
6.84E-06 |
3.59E-05 |
|
| Knife clam | 5.07E-09 |
5.07E-09 |
1.07E-05 |
7.48E-06 |
1.97E-05 |
6.43E-05 |
5.07E-09 |
1.02E-04 |
|
| Mangrove oyster | 8.83E-06 |
5.07E-09 |
5.07E-09 |
5.07E-09 |
9.07E-06 |
9.46E-06 |
9.12E-06 |
3.65E-05 |
|
| Bonny | Bloody Cockle | 5.07E-09 |
5.72E-05 |
6.62E-05 |
5.07E-09 |
1.33E-05 |
5.07E-09 |
6.42E-05 |
2.01E-04 |
| Donax clam | 5.07E-09 |
5.07E-09 |
6.50E-05 |
5.07E-09 |
5.07E-09 |
6.44E-05 |
6.63E-05 |
1.96E-04 |
|
| Knife clam | 5.07E-09 |
5.07E-09 |
6.26E-05 |
5.07E-09 |
8.41E-06 |
6.68E-05 |
6.24E-05 |
2.00E-04 |
|
| Mangrove oyster | 5.07E-09 |
6.13E-06 |
5.46E-05 |
5.07E-09 |
5.78E-06 |
1.04E-05 |
2.95E-05 |
1.06E-04 |
|
| Ibeno | Bloody Cockle | 5.07E-09 |
8.26E-06 |
1.20E-05 |
1.96E-06 |
1.24E-05 |
1.63E-05 |
6.62E-06 |
5.75E-05 |
| Donax clam | 7.40E-06 |
5.07E-09 |
6.62E-06 |
2.28E-05 |
1.12E-05 |
6.13E-06 |
1.24E-05 |
6.65E-05 |
|
| Knife clam | 5.07E-09 |
5.07E-09 |
1.19E-05 |
7.72E-06 |
1.21E-05 |
5.07E-09 |
1.56E-05 |
4.73E-05 |
|
| Mangrove oyster | 1.10E-05 |
2.11E-06 |
1.30E-05 |
1.20E-21 |
2.35E-05 |
2.73E-05 |
1.65E-05 |
9.35E-05 |
|
| Iko Town | Bloody Cockle | 1.13E-05 |
5.07E-09 |
1.24E-04 |
8.40E-06 |
9.00E-06 |
1.14E-05 |
9.83E-06 |
1.74E-04 |
| Donax clam | 2.38E-06 |
5.07E-09 |
7.40E-05 |
8.68E-06 |
2.60E-06 |
9.37E-06 |
9.29E-06 |
1.06E-04 |
|
| Knife clam | 5.07E-09 |
8.18E-06 |
1.13E-04 |
1.10E-05 |
8.29E-06 |
5.07E-09 |
1.22E-05 |
1.52E-04 |
|
| Mangrove oyster | 3.28E-05 |
1.24E-05 |
6.76E-05 |
5.07E-09 |
9.09E-06 |
9.83E-06 |
1.94E-05 |
1.51E-04 |
|
| BaA: Benzo(a)anthracene, Chry: Chrysene, BbF: Benzo(b)fluoranthene, BkF: Benzo(k)fluoranthene, BaP: Benzo(a)pyrene, DahA: Dibenzo(a,h)anthracene and IP: Indo(1,2,3-cd)pyrene | |||||||||
For instance one out of 100,000 chances of consumers in Iko Town and Andoni, one out of 100,000 and 3 out of 100,000 chances of consumers were likely to suffer cancer-related illness in their lifetime due to benzo(a)anthracene exposure. Of concern also is the carcinogenic values of benzo(b)fluoranthene, benzo(a)pyrene and indo (1,2,3-cd) pyrene in almost all bivalve samples from the study locations were higher than the USEPA18 safety limit of 1.0×10–6. These values are similar to the report of Tongo et al.26 on the human risk of PAHs in fish and shellfish in Bonny River State. The cumulative lifetime cancer risk (ΣLCR) also indicated values higher than the one in one million chance as outlined by USEPA18, which suggest that lifetime exposure to some of these PAHs congeners and their cumulative effect through bivalve consumption would result in cancer risk. This shows that consumption of bivalve shellfish could result in potential cancer risk. Several studies1,26 have reported excess cancer risk (ECR) due to oral exposure of seafood higher than the regulatory permissible limit. However, Qu et al.27, noted that this model of carcinogenic risk assessment of contaminants does not assume any threshold for effects and as such it is believed (but not proved) that the benzo(a)pyrene slope factor (7.39 mg/ug/day) usually overestimate the actual cancer incidence associated with low-dose exposure to environmental pollutants but this study provides rationale on the need to fully evaluate the risk of PAHs in bivalve to safeguard the health of consumers. Potential health risk of PAHs consumption cancer potency evaluations of environmental matrices is a vital component of cancer risk assessments. Several PAHs have shown carcinogenic effects in experimental animals and it has been concluded that benzo(a)pyrene is carcinogenic to humans.
CONCLUSION
Polycyclic Aromatic Hydrocarbon (PAHs) remains a potential food safety hazard associated with seafood harvested from the brackish waters of the Niger Delta, this is particularly due to anthropogenic activities such as artisanal refining of petroleum products, oil spillage, gas flaring and well bush burning that are unabatedly prevalence in this region. The present study showed elevated tissue concentration of polycyclic aromatic hydrocarbons in bivalve shellfish far above the legal maximum limits allowed for food safety. The estimated values for human health risk assessment revealed a non-carcinogenic value and hazard index less than one for non-carcinogenic PAHs indicating that consumers are not likely to experience any significant health risk through the consumption of 48 g day–1 of bivalve samples. However, the non-biodegradable nature and the resultant bioaccumulation and bio-magnification along the food chain possess a tremendous health challenge to bivalve shellfish consumers. Also, risk value for carcinogenic PAHs and cumulative cancer indices at some study locations were higher than the stipulated one in one million (1.0×10–6) chances as stipulated by regulatory bodies which imply that carcinogenic effects were more likely due to consumption of 48 g day–1 of bivalve molluscs with PAHs contaminants. Further studies on food safety risk assessment be extended to more food matrices more importantly edible benthic macrofauna, shrimps, crabs, catfish, sea snails and other important consumable seafood. This will help to generate enough evidence for regulatory and advisory purposes.
SIGNIFICANCE STATEMENT
The accumulated Polycyclic Aromatic Hydrocarbons (PAHs) in the tissues of bivalve shellfish is typically above the legal regulated limit considered safe for human consumption. The potential health risk of PAHs exposure is cancer. Food consumption is the main source of Polycyclic Aromatic Hydrocarbon (PAHs) intake, thus highlighting the importance of research on PAHs in food and the development of mitigation strategies to reduce their contents in food. This study exposes the consequences of blatant and consistent abuse of the environment by International oil and gas companies operating in the Niger Delta Region of Nigeria and the negligence of government and regulatory bodies in addressing the menace. This study discovered that bivalve shellfish harvested from the coastal waters of the Niger Delta contains a significant amount of Polycyclic Aromatic Hydrocarbons (PAHs) which is adjudged to be at toxic levels from a regulatory standpoint. Therefore bivalve shellfish harvested from these locations can induce potential deleterious health effects to the consumers except something urgent is done by government and international oil and gas companies operating in this area.
REFERENCES
- Bandowe, B.A.M., M. Bigalke, L. Boamah, E. Nyarko, F.K. Saalia and W. Wilcke, 2014. Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): Bioaccumulation and health risk assessment. Environ. Int., 65: 135-146.
- Wang, D.Q., Y.X. Yu, X.Y. Zhang, S.H. Zhang and Y.P. Pang et al., 2012. Polycyclic aromatic hydrocarbons and organochlorine pesticides in fish from Taihu lake: Their levels, sources and biomagnification. Ecotoxicol. Environ. Saf., 82: 63-70.
- Beg, M.U., B. Gevao, N. Al-Jandal, K.R. Beg, S.A. Butt, L.N. Ali and M. Al-Hussaini, 2009. Polycyclic aromatic hydrocarbons in three varieties of fish from Kuwait Bay. Polycyclic Aromat. Compd., 29: 75-89.
- Skupińska, K., I. Misiewicz and T. Kasprzycka-Guttman, 2004. Polycyclic aromatic hydrocarbons: Physicochemical properties, environmentat appearance and impact on living organisms. Acta Poloniae Pharmaceutica, 61: 233-240.
- Brown, J.N. and B.M. Peake, 2006. Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Sci. Total Environ., 359: 145-155.
- Gosling, E.M., 2003. Bivalve Molluscs: Biology, Ecology and Culture. Blackwell Publishing, Hoboken, New Jersey, ISBN-13: 9780852382349, Pages: 443.
- Gopalsamy, I., M. Arumugam, K. Saravanan and B. Thangavel, 2014. Nutritional value of marine bivalve, Donax cuneatus (Linnaeus, 1758) from cuddalore coastal waters, Southeast Coast of India. Inventi Impact: Life Style, 1: 15-19.
- Sarkar, S.K., H. Cabral, M. Chatterjee, I. Cardoso, A.K. Bhattacharya, K.K. Satpathy and M.A. Alam, 2008. Biomonitoring of heavy metals using the bivalve molluscs in Sunderban mangrove wetland, Northeast Coast of Bay of Bengal (India): Possible risks to human health. Clean: Soil Air Water, 36: 187-194.
- Espana, M.S.A., E.M.R. Rodríguez and C.D. Romero, 2007. Comparison of mineral and trace element concentrations in two molluscs from the Strait of Magellan (Chile). J. Food Compos. Anal., 20: 273-279.
- Orban, E., G. di Lena, T. Nevigato, I. Casini, A. Marzetti and R. Caproni, 2002. Seasonal changes in meat content, condition index and chemical composition of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem., 77: 57-65.
- FAO and WHO, 2011. Food and agriculture organisation of the United Nations. Report of the joint FAO/WHO expert consultation on the risk and benefit of fish consumption. FAO Fisheries and Aquaculture Report No. 978 Rome.
- Huss, H.H., L. Ababouch and L. Gram, 2003. Assessment and Management of Seafood Safety and Quality. FAO, Rome, Italy, ISBN: 92-5-104954-8, Pages: 230.
- Lees, D., 2000. Viruses and bivalve shellfish. Int. J. Food Microbiol., 59: 81-116.
- Anifowose, B., D. Lawler, D van der Horst and L. Chapman, 2014. Evaluating interdiction of oil pipelines at river crossings using environmental impact assessments. Area, 46: 4-17.
- Amnesty International, 2018. Negligence in the Niger Delta; decoding shell and Eni’s poor record on oil spills. Amnesty International Publication London UK.
- Zabbey, N., 2004. Impacts of extractive industries on the biodiversity of the Niger Delta Region, Nigeria. Proceedings of the National Workshop on Coastal and Marine Biodiversity Management, September 7-9, 2004, Calabar, Cross River State, Nigeria.
- Udotong, J.I.R., U.P. Udoudo and I.R. Udotong, 2017. Effects of oil and gas exploration and production activities on production and management of seafood in Akwa Ibom State, Nigeria. J. Environ. Chem. Ecotoxicol., 9: 20-42.
- Cheung, K.C., H.M. Leung, K.Y. Kong and M.H. Wong, 2007. Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment. Chemosphere, 66: 460-468.
- Yakubu, O.H., 2017. Addressing environmental health problems in ogoniland through implementation of united nations environment program recommendations: Environmental management strategies. Environments.
- Ukwo, S., C. Ezeama and K. Abasiekong, 2020. Hazard Indices and human health risks associated with toxic element contaminants in bivalve shellfish from Niger Delta, Nigeria. Arch. Ecotoxicol., 2: 61-68.
- Guo, W., M. He, Z. Yang, C. Lin, X. Quan and H. Wang, 2007. Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere, 68: 93-104.
- Ramesh, A., S.A. Walker, D.B. Hood, M.D. Guillén, K. Schneider and E.H. Weyand, 2004. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol., 23: 301-333.
- Nasr, I.N., Neveen, H.A. EL-Enaen and T.A. Yosef, 2012. Study of some polycyclic aromatic hydrocarbons residues in fish at sharkia governorate markets in relation to public health. Global Vet., 8: 670-675.
- Zuloaga, O., A. Prieto, A. Usobiaga, S.K. Sarkar and M. Chatterjee et al., 2009. Polycyclic aromatic hydrocarbons in intertidal marine bivalves of sunderban mangrove wetland, India: An approach to bioindicator species. Water Air Soil Pollut., 201: 305-318.
- Mohammed, Z., J.C. Akan, L.I. Bukar and A.M. Idi, 2017. Cancer and non-cancer risk associated with PAHs exposure from consumption of fish from Komadugu River Basin, Yobe State, Nigeria. J. Aquat. Pollut. Toxicol., Vol. 1.
- Tongo, I., E.E. Etor and L. Ezemonye, 2018. Human health risk assessment of PAHs in fish and shellfish from Amariaria Community, Bonny River, Nigeria. J. Appl. Sci. Environ. Manage., 22: 731-736.
- Qu, C., B. Li, H. Wu, S. Wang and J.P. Giesy, 2015. Multi-pathway assessment of human health risk posed by polycyclic aromatic hydrocarbons. Environ. Geochem. Health, 37: 587-601.
How to Cite this paper?
APA-7 Style
Ukwo,
S.P., Edima-Nyah,
A.P., Udo,
I.I. (2023). Hazard Indices and Human Health Risks Associated with PAHs Exposure from Consumption of Bivalve Shellfish from Niger Delta, Nigeria. Trends in Applied Sciences Research, 18(1), 19-28. https://doi.org/10.3923/tasr.2023.19.28
ACS Style
Ukwo,
S.P.; Edima-Nyah,
A.P.; Udo,
I.I. Hazard Indices and Human Health Risks Associated with PAHs Exposure from Consumption of Bivalve Shellfish from Niger Delta, Nigeria. Trends Appl. Sci. Res 2023, 18, 19-28. https://doi.org/10.3923/tasr.2023.19.28
AMA Style
Ukwo
SP, Edima-Nyah
AP, Udo
II. Hazard Indices and Human Health Risks Associated with PAHs Exposure from Consumption of Bivalve Shellfish from Niger Delta, Nigeria. Trends in Applied Sciences Research. 2023; 18(1): 19-28. https://doi.org/10.3923/tasr.2023.19.28
Chicago/Turabian Style
Ukwo, Sunday, Peter, Anne Peter Edima-Nyah, and Ifiok Ikpeme Udo.
2023. "Hazard Indices and Human Health Risks Associated with PAHs Exposure from Consumption of Bivalve Shellfish from Niger Delta, Nigeria" Trends in Applied Sciences Research 18, no. 1: 19-28. https://doi.org/10.3923/tasr.2023.19.28

This work is licensed under a Creative Commons Attribution 4.0 International License.


